Langerin-mediated internalization of a modified peptide routes antigens to early endosomes and enhances cross-presentation by human Langerhans cells
- PMID: 26456691
- PMCID: PMC5380941
- DOI: 10.1038/cmi.2015.87
Langerin-mediated internalization of a modified peptide routes antigens to early endosomes and enhances cross-presentation by human Langerhans cells
Abstract
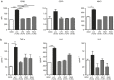
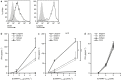
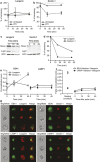
The potential of the skin immune system to generate immune responses is well established, and the skin is actively exploited as a vaccination site. Human skin contains several antigen-presenting cell subsets with specialized functions. In particular, the capacity to cross-present exogenous antigens to CD8+ T cells is of interest for the design of effective immunotherapies against viruses or cancer. Here, we show that primary human Langerhans cells (LCs) were able to cross-present a synthetic long peptide (SLP) to CD8+ T cells. In addition, modification of this SLP using antibodies against the receptor langerin, but not dectin-1, further enhanced the cross-presenting capacity of LCs through routing of internalized antigens to less proteolytic early endosome antigen 1+ early endosomes. The potency of LCs to enhance CD8+ T-cell responses could be further increased through activation of LCs with the toll-like receptor 3 ligand polyinosinic:polycytidylic acid (pI:C). Altogether, the data provide evidence that human LCs are able to cross-present antigens after langerin-mediated internalization. Furthermore, the potential for antigen modification to target LCs specifically provides a rationale for generating effective anti-tumor or anti-viral cytotoxic T lymphocyte responses.
Figures


References
-
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev 2004; 199: 9–26. - PubMed
-
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12: 557–569. - PubMed
-
- Valladeau J, Dezutter-Dambuyant C, Saeland S. Langerin/CD207 sheds light on formation of birbeck granules and their possible function in Langerhans cells. Immunol Res 2003; 28: 93–107. - PubMed
-
- Furio L, Briotet I, Journeaux A, Billard H, Peguet-Navarro J. Human Langerhans cells are more efficient than CD14(-)CD1c(+) dermal dendritic cells at priming naive CD4(+) T cells. J Invest Dermatol 2010; 130: 1345–1354. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

