Clinical Signs and Diagnosis of the Canine Primary Glaucomas
- PMID: 26456752
- PMCID: PMC4862370
- DOI: 10.1016/j.cvsm.2015.06.006
Clinical Signs and Diagnosis of the Canine Primary Glaucomas
Abstract
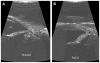
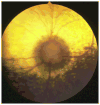
The diagnosis of glaucoma is highly dependent on a working understanding of the clinical signs and available diagnostic procedures. Clinical signs may be attributable to increased intraocular pressure and/or complex alterations in the physiology or molecular biology of the anterior segment, retinal ganglion cells, and optic nerve. Many diagnostic procedures seek to more fully characterize these alterations and to identify which clinical features increase the risk of overt primary angle closure glaucoma (PACG) occurring. Considerable progress has been made in identifying the anatomic features that predispose an eye to PACG, and in elucidating the role of reverse pupillary block.
Keywords: Canine; Glaucoma; Intraocular pressure; Ocular imaging; Optic neuropathy; Tonometry.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

















References
-
- Spaeth GL, Waisbourd M. Definitions: what is glaucoma worldwide? In: Shaarawy TM, Sherwood MB, Hitchings RA, et al., editors. Glaucoma: medical diagnosis & therapy. St Louis (MO): Elsevier Ltd; 2015. pp. 311–24.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

