Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: An NRG oncology/gynecologic oncology group study
- PMID: 26456812
- PMCID: PMC4698796
- DOI: 10.1016/j.ygyno.2015.10.003
Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: An NRG oncology/gynecologic oncology group study
Abstract
Objectives: Changes in cognitive function have been identified in and reported by many cancer survivors. These changes have the potential to impact patient quality of life and functional ability. This prospective longitudinal study was designed to quantify the incidence of change in cognitive function in newly diagnosed ovarian cancer patients throughout and following primary chemotherapy.
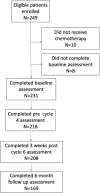
Methods: Eligible patients had newly diagnosed, untreated ovarian cancer and had planned to receive chemotherapy. Web-based and patient reported cognitive assessments and quality of life questionnaires were conducted prior to chemotherapy, prior to cycle four, after cycle six, and six months after completion of primary therapy.
Results: Two-hundred-thirty-one evaluable patients entered this study between May 2010 and October 2011. At the cycle 4 time point, 25.2% (55/218) of patients exhibited cognitive impairment in at least one domain. At the post-cycle 6 and 6-month follow up time points, 21.1% (44/208) and 17.8% (30/169) of patients, respectively, demonstrated impairment in at least one domain of cognitive function. There were statistically significant, but clinically small, improvements in processing speed (p<0.001) and attention (p<0.001) but not in motor response time (p=0.066), from baseline through the six-month follow up time period.
Conclusions: This was a large, prospective study designed to measure cognitive function in ovarian cancer. A subset of patients had evidence of cognitive decline from baseline during chemotherapy treatment in this study as measured by the web-based assessment; however, changes were generally limited to no more than one domain.
Keywords: Chemotherapy; Cognitive function; Ovarian cancer; Prospective trial; Quality of life.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Lisa Hess was an employee of Indiana University during the conduct and completion of this study. After completion of this work she accepted a position at Eli Lilly and Co. Dr. Robert Mannel received per capita funding for performing research through the Gynecologic Oncology Group, NCI. He also served on the Advisory Board for clinical trial development with Amgen, Advaxis, MedImmune, Astra Zeneca, Oxigene and Endocyte.
All other co-authors have no conflicts of interest to declare.
References
-
- Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. - PubMed
-
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–2239. - PubMed
-
- Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. - PubMed
-
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. - PubMed
-
- Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical