Long-term safety and efficacy of insulin degludec in the management of type 2 diabetes
- PMID: 26457056
- PMCID: PMC4598200
- DOI: 10.2147/DMSO.S54953
Long-term safety and efficacy of insulin degludec in the management of type 2 diabetes
Abstract
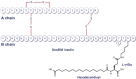
Insulin degludec (IDeg) is a novel antiglycemic agent belonging to the therapeutic class of ultra-long duration basal insulin analogs. Its half-life and duration of action are 25 hours and 42 hours, respectively. This pharmacodynamic profile leads to a strict dosing schedule, ie, IDeg is injected at the same time each day to ensure optimal biological action and consistent glycemic control. According to the literature, IDeg provides glycemic control and nocturnal hypoglycemia reduction comparable with other long-acting analogs in type 2 diabetes mellitus. The risk of severe hypoglycemic episodes seems also to be reduced when using IDeg therapy; however, long-term follow-up is warranted for monitoring of possible but relatively infrequent adverse events. IDeg is also available in combination with aspart insulin and with liraglutide. The above preparations have been approved by the European Medicines Agency and other national health authorities. In 2012, the US Food and Drug Administration asked for a complementary study on IDeg-associated cardiovascular risk. Future prospective evaluation of large cohorts of patients with type 2 diabetes mellitus treated with IDeg, with long-term follow-up, can provide further relevant information on the safety of IDeg therapy.
Keywords: HbA1c; basal insulin analog; degludec insulin; hypoglycemia; safety; type 2 diabetes mellitus.
Figures
References
-
- Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–2148. - PubMed
-
- Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;2:CD005613. - PubMed
-
- Inzucchi Silvio E, Bergenstal Richard M, Buse John B, et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

