Recognition Code of ZNF191(243-368) and Its Interaction with DNA
- PMID: 26457075
- PMCID: PMC4592708
- DOI: 10.1155/2015/416751
Recognition Code of ZNF191(243-368) and Its Interaction with DNA
Abstract
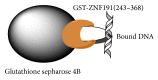
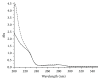
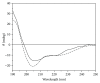
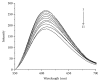
ZNF191(243-368) is the C-terminal region of ZNF191 which contains a putative DNA-binding domain of four Cys2His2 zinc finger motifs. In this study, an expression vector of a fusion protein of ZNF191(243-368) with glutathione-S-transferase (GST) was constructed and transformed into Escherichia coli BL21. The fusion protein GST-ZNF191(243-368) was expressed using this vector to investigate the protein-DNA binding reaction through an affinity selection strategy on the basis of the binding quality of the zinc finger domain. Results showed that ZNF191(243-368) can selectively bind with sequences and react with genes which contain an AGGG core. However, the recognition mechanism of Cys2His2 zinc finger proteins to DNA warrants further investigation.
Figures


Similar articles
-
Effect of His-Tag on Expression, Purification, and Structure of Zinc Finger Protein, ZNF191(243-368).Bioinorg Chem Appl. 2016;2016:8206854. doi: 10.1155/2016/8206854. Epub 2016 Jul 20. Bioinorg Chem Appl. 2016. PMID: 27524954 Free PMC article.
-
Expression, purification, and characterization of N-terminal His-tagged proteins with mutations in zinc finger 3 of zinc finger protein ZNF191(243-368).Prep Biochem Biotechnol. 2018;48(10):914-919. doi: 10.1080/10826068.2018.1514518. Epub 2018 Oct 8. Prep Biochem Biotechnol. 2018. PMID: 30296200
-
A transcript profiling approach reveals the zinc finger transcription factor ZNF191 is a pleiotropic factor.BMC Genomics. 2009 May 22;10:241. doi: 10.1186/1471-2164-10-241. BMC Genomics. 2009. PMID: 19463170 Free PMC article.
-
DNA recognition by Cys2His2 zinc finger proteins.Annu Rev Biophys Biomol Struct. 2000;29:183-212. doi: 10.1146/annurev.biophys.29.1.183. Annu Rev Biophys Biomol Struct. 2000. PMID: 10940247 Review.
-
Design and selection of novel Cys2His2 zinc finger proteins.Annu Rev Biochem. 2001;70:313-40. doi: 10.1146/annurev.biochem.70.1.313. Annu Rev Biochem. 2001. PMID: 11395410 Review.
Cited by
-
Effect of His-Tag on Expression, Purification, and Structure of Zinc Finger Protein, ZNF191(243-368).Bioinorg Chem Appl. 2016;2016:8206854. doi: 10.1155/2016/8206854. Epub 2016 Jul 20. Bioinorg Chem Appl. 2016. PMID: 27524954 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

