Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing
- PMID: 26457553
- PMCID: PMC4624522
- DOI: 10.1038/nn.4131
Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing
Abstract
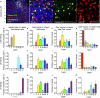
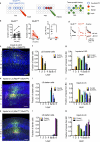
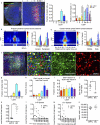
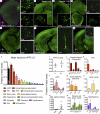
Information processing in neocortical circuits requires integrating inputs over a wide range of spatial scales, from local microcircuits to long-range cortical and subcortical connections. We used rabies virus-based trans-synaptic tracing to analyze the laminar distribution of local and long-range inputs to pyramidal neurons in the mouse barrel cortex and medial prefrontal cortex (mPFC). In barrel cortex, we found substantial inputs from layer 3 (L3) to L6, prevalent translaminar inhibitory inputs, and long-range inputs to L2/3 or L5/6 preferentially from L2/3 or L5/6 of input cortical areas, respectively. These layer-specific input patterns were largely independent of NMDA receptor function in the recipient neurons. mPFC L5 received proportionally more long-range inputs and more local inhibitory inputs than barrel cortex L5. Our results provide new insight into the organization and development of neocortical networks and identify important differences in the circuit organization in sensory and association cortices.
Figures




References
-
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. - PubMed
-
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. - PubMed
-
- Fuster JM. The prefrontal cortex : anatomy, physiology, and neuropsychology of the frontal lobe. Academic Press; Philadelphia: 2008.
-
- Douglas RJM, Martin KAC, Whitteridge D. A Canonical Microcircuit for the Neocortex. Neural Computation. 1989;1:480–488.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

