Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study
- PMID: 26457568
- PMCID: PMC4684979
- DOI: 10.1080/15504263.2015.1100471
Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study
Abstract
Objective: Depression among methamphetamine users is more prevalent in females than males, but gender-specific treatment options for this comorbidity have not been described. Reduced brain phosphocreatine levels have been shown to be lower in female methamphetamine users compared to males, and, of relevance, studies have demonstrated an association between treatment-resistant depression and reduced brain phosphocreatine concentrations. The nutritional supplement creatine monohydrate has been reported to reduce symptoms of depression in female adolescents and adults taking antidepressants, as well as to increase brain phosphocreatine in healthy volunteers. Therefore, the purpose of this pilot study was to investigate creatine monohydrate as a treatment for depression in female methamphetamine users.
Methods: Fourteen females with depression and comorbid methamphetamine dependence were enrolled in an 8-week open label trial of 5 g of daily creatine monohydrate and of these 14, 11 females completed the study. Depression was measured using the Hamilton Depression Rating Scale (HAMD) and brain phosphocreatine levels were measured using phosphorus magnetic resonance spectroscopy pre- and post-creatine treatment. Secondary outcome measures included anxiety symptoms, measured with the Beck Anxiety Inventory (BAI), as well as methamphetamine use, monitored by twice weekly urine drug screens and self-reported use.
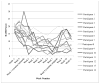
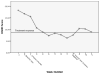
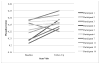
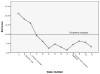
Results: The results of a linear mixed effects repeated measures model showed significantly reduced HAMD and BAI scores as early as week 2 when compared to baseline scores. This improvement was maintained through study completion. Brain phosphocreatine concentrations were higher at the second phosphorus magnetic resonance spectroscopy scan compared to the baseline scan; Mbaseline = 0.223 (SD = 0.013) vs. Mpost-treatment = 0.233 (SD = 0.009), t (9) = 2.905, p <.01, suggesting that creatine increased phosphocreatine levels. Also, a reduction in methamphetamine positive urine drug screens of greater than 50% was observed by week 6. Finally, creatine was well tolerated and adverse events that were related to gastrointestinal symptoms and muscle cramping were determined as possibly related to creatine.
Conclusions: The current study suggests that creatine treatment may be a promising therapeutic approach for females with depression and comorbid methamphetamine dependence. This study is registered on clinicaltrials.gov (NCT01514630).
Keywords: co-occurring; comorbidity; depression; methamphetamine dependence; neuroimaging; substance use disorders; women's health.
Conflict of interest statement
Dr. Perry Renshaw serves as a consultant to Kyowa Hakko, Tal Medical and Ridge Diagnostics. He has received research support from GlaxoSmithKline and Roche. Dr. Perry Renshaw is an inventor on a patent application that has been assigned to the University of Utah, which describes the use of creatine as a treatment for depressive disorders. All other authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study.J Affect Disord. 2011 Dec;135(1-3):354-61. doi: 10.1016/j.jad.2011.07.010. Epub 2011 Aug 9. J Affect Disord. 2011. PMID: 21831448 Free PMC article. Clinical Trial.
-
Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study.Arch Gen Psychiatry. 2005 Apr;62(4):444-52. doi: 10.1001/archpsyc.62.4.444. Arch Gen Psychiatry. 2005. PMID: 15809412
-
A review of treatment options for co-occurring methamphetamine use disorders and depression.J Addict Nurs. 2015 Jan-Mar;26(1):14-23; quiz E1. doi: 10.1097/JAN.0000000000000058. J Addict Nurs. 2015. PMID: 25761159 Free PMC article. Review.
-
Gender differences in the effect of tobacco use on brain phosphocreatine levels in methamphetamine-dependent subjects.Am J Drug Alcohol Abuse. 2015;41(4):281-9. doi: 10.3109/00952990.2015.1019673. Epub 2015 Apr 14. Am J Drug Alcohol Abuse. 2015. PMID: 25871447 Free PMC article.
-
Structural and metabolic brain changes in the striatum associated with methamphetamine abuse.Addiction. 2007 Apr;102 Suppl 1:16-32. doi: 10.1111/j.1360-0443.2006.01782.x. Addiction. 2007. PMID: 17493050 Review.
Cited by
-
Creatine Supplementation in Women's Health: A Lifespan Perspective.Nutrients. 2021 Mar 8;13(3):877. doi: 10.3390/nu13030877. Nutrients. 2021. PMID: 33800439 Free PMC article. Review.
-
Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition.Nutrients. 2022 Mar 15;14(6):1230. doi: 10.3390/nu14061230. Nutrients. 2022. PMID: 35334887 Free PMC article. Review.
-
Aerobic training and vitamin E administration ameliorates cardiac apoptosis markers in rats exposed to methamphetamine.Eur J Transl Myol. 2023 Dec 11;33(4):12112. doi: 10.4081/ejtm.2023.12112. Eur J Transl Myol. 2023. PMID: 38112583 Free PMC article.
-
Sex-Based Impact of Creatine Supplementation on Depressive Symptoms, Brain Serotonin and SSRI Efficacy in an Animal Model of Treatment-Resistant Depression.Int J Mol Sci. 2021 Jul 30;22(15):8195. doi: 10.3390/ijms22158195. Int J Mol Sci. 2021. PMID: 34360959 Free PMC article.
-
Creatine as a Therapeutic Target in Alzheimer's Disease.Curr Dev Nutr. 2023 Sep 29;7(11):102011. doi: 10.1016/j.cdnut.2023.102011. eCollection 2023 Nov. Curr Dev Nutr. 2023. PMID: 37881206 Free PMC article. Review.
References
-
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Research Bulletin. 2008;76(4):329–343. - PubMed
-
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: Has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. - PubMed
-
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
