CD and NMR investigation of collagen peptides mimicking a pathological Gly-Ser mutation and a natural interruption in a similar highly charged sequence context
- PMID: 26457583
- PMCID: PMC4815337
- DOI: 10.1002/pro.2828
CD and NMR investigation of collagen peptides mimicking a pathological Gly-Ser mutation and a natural interruption in a similar highly charged sequence context
Abstract
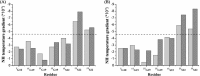
Even a single Gly substitution in the triple helix domain of collagen leads to pathological conditions while natural interruptions are suggested to play important functional roles. Two peptides-one mimicking a pathological Gly-Ser substitution (ERSEQ) and the other one modeling a similar natural interruption sequence (DRSER)-are designed to facilitate the comparison for elucidating the molecular basis of their different biological roles. CD and NMR investigation of peptide ERSEQ indicates a reduction of the thermal stability and disruption of hydrogen bonding at the Ser mutation site, providing a structural basis of the OI disease resulting from the Gly-Ser mutation in the highly charged RGE environment. Both CD and NMR real-time folding results indicate that peptide ERSEQ displays a comparatively slower folding rate than peptide DRSER, suggesting that the Gly-Ser mutation may lead to a larger interference in folding than the natural interruption in a similar RSE context. Our studies suggest that unlike the rigid GPO environment, the abundant R(K)GE(D) motif may provide a more flexible sequence environment that better accommodates mutations as well as interruptions, while the electrostatic interactions contribute to its stability. These results shed insight into the molecular features of the highly charged motif and may aid the design of collagen biomimetic peptides containing important biological sites.
Keywords: CD; NMR; collagen; interruption; mutation.
© 2015 The Protein Society.
Figures




References
-
- Ramachandran GN, Kartha G (1955) Structure of collagen. Nature 176:593–595. - PubMed
-
- Rich A, Crick FH (1955) The structure of collagen. Nature 176:915–916. - PubMed
-
- van der Rest M, Garrone R (1991) Collagen family of proteins. FASEB J 5:2814–2823. - PubMed
-
- Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Korkko J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila‐Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH (2007) Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 28:209–221. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

