Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency
- PMID: 26457731
- PMCID: PMC4639965
- DOI: 10.1172/JCI80477
Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency
Erratum in
-
Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency.J Clin Invest. 2016 Nov 1;126(11):4389. doi: 10.1172/JCI91162. Epub 2016 Nov 1. J Clin Invest. 2016. PMID: 27801680 Free PMC article. No abstract available.
Abstract
Patients with mutations of the recombination-activating genes (RAG) present with diverse clinical phenotypes, including severe combined immune deficiency (SCID), autoimmunity, and inflammation. However, the incidence and extent of immune dysregulation in RAG-dependent immunodeficiency have not been studied in detail. Here, we have demonstrated that patients with hypomorphic RAG mutations, especially those with delayed-onset combined immune deficiency and granulomatous/autoimmune manifestations (CID-G/AI), produce a broad spectrum of autoantibodies. Neutralizing anti-IFN-α or anti-IFN-ω antibodies were present at detectable levels in patients with CID-G/AI who had a history of severe viral infections. As this autoantibody profile is not observed in a wide range of other primary immunodeficiencies, we hypothesized that recurrent or chronic viral infections may precipitate or aggravate immune dysregulation in RAG-deficient hosts. We repeatedly challenged Rag1S723C/S723C mice, which serve as a model of leaky SCID, with agonists of the virus-recognizing receptors TLR3/MDA5, TLR7/-8, and TLR9 and found that this treatment elicits autoantibody production. Altogether, our data demonstrate that immune dysregulation is an integral aspect of RAG-associated immunodeficiency and indicate that environmental triggers may modulate the phenotypic expression of autoimmune manifestations.
Figures
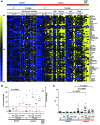

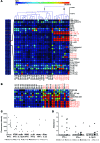

References
-
- Pessach I, Walter J, Notarangelo LD. Recent advances in primary immunodeficiencies: identification of novel genetic defects and unanticipated phenotypes. Pediatr Res. 2009;65(5 pt 2):3R–12R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

