Pro-nerve growth factor in the ovary and human granulosa cells
- PMID: 26457789
- PMCID: PMC4760111
- DOI: 10.1515/hmbci-2015-0028
Pro-nerve growth factor in the ovary and human granulosa cells
Abstract
Background: Pro-nerve growth factor must be cleaved to generate mature NGF, which was suggested to be a factor involved in ovarian physiology and pathology. Extracellular proNGF can induce cell death in many tissues. Whether extracellular proNGF exists in the ovary and may play a role in the death of follicular cells or atresia was unknown.
Materials and methods: Immunohistochemistry of human and rhesus monkey ovarian sections was performed. IVF-derived follicular fluid and human granulosa cells were studied by RT-PCR, qPCR, Western blotting, ATP- and caspase-assays.
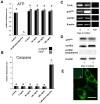
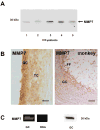
Results and conclusion: Immunohistochemistry of ovarian sections identified proNGF in granulosa cells and Western blotting of human isolated granulosa cells confirmed the presence of proNGF. Ovarian granulosa cells thus produce proNGF. Recombinant human proNGF even at high concentrations did not affect the levels of ATP or the activity of caspase 3/7, indicating that in granulosa cells proNGF does not induce death. In contrast, mature NGF, which was detected previously in follicular fluid, may be a trophic molecule for granulosa cells with unexpected functions. We found that in contrast to proNGF, NGF increased the levels of the transcription factor early growth response 1 and of the enzyme choline acetyl-transferase. A mechanism for the generation of mature NGF from proNGF in the follicular fluid may be extracellular enzymatic cleavage. The enzyme MMP7 is known to cleave proNGF and was identified in follicular fluid and as a product of granulosa cells. Thus the generation of NGF in the ovarian follicle may depend on MMP7.
Figures



Similar articles
-
Cleavage of poly (ADP-ribose) polymerase-1 is involved in the process of porcine ovarian follicular atresia.Anim Reprod Sci. 2013 May;138(3-4):282-91. doi: 10.1016/j.anireprosci.2013.02.025. Epub 2013 Mar 14. Anim Reprod Sci. 2013. PMID: 23522430
-
The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor.J Neurochem. 2004 May;89(3):581-92. doi: 10.1111/j.1471-4159.2004.02360.x. J Neurochem. 2004. PMID: 15086515
-
Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: implications for physiology and pathology.Hum Reprod. 2014 Mar;29(3):555-67. doi: 10.1093/humrep/det422. Epub 2013 Nov 27. Hum Reprod. 2014. PMID: 24287819
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
Autophagy in Ovarian Follicular Development and Atresia.Int J Biol Sci. 2019 Jan 29;15(4):726-737. doi: 10.7150/ijbs.30369. eCollection 2019. Int J Biol Sci. 2019. PMID: 30906205 Free PMC article. Review.
Cited by
-
Retinoic acid promotes in vitro follicle activation in the cat ovary by regulating expression of matrix metalloproteinase 9.PLoS One. 2018 Aug 24;13(8):e0202759. doi: 10.1371/journal.pone.0202759. eCollection 2018. PLoS One. 2018. PMID: 30142172 Free PMC article.
-
CD73's Potential as an Immunotherapy Target in Gastrointestinal Cancers.Front Immunol. 2020 Apr 15;11:508. doi: 10.3389/fimmu.2020.00508. eCollection 2020. Front Immunol. 2020. PMID: 32351498 Free PMC article. Review.
-
Nerve Growth Factor: A Dual Activator of Noradrenergic and Cholinergic Systems of the Rat Ovary.Front Endocrinol (Lausanne). 2021 Feb 25;12:636600. doi: 10.3389/fendo.2021.636600. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33716987 Free PMC article.
-
ProNGF processing in adult rat tissues and bioactivity of NGF prodomain peptides.FEBS Open Bio. 2024 Apr;14(4):643-654. doi: 10.1002/2211-5463.13768. Epub 2024 Mar 1. FEBS Open Bio. 2024. PMID: 38429912 Free PMC article.
-
Human Luteinized Granulosa Cells-A Cellular Model for the Human Corpus Luteum.Front Endocrinol (Lausanne). 2019 Jul 9;10:452. doi: 10.3389/fendo.2019.00452. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31338068 Free PMC article. Review.
References
-
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. - PubMed
-
- Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med. 2012;209:2291–2305. - PMC - PubMed
-
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. - PubMed
-
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
