Dynamic m(6)A mRNA methylation directs translational control of heat shock response
- PMID: 26458103
- PMCID: PMC4851248
- DOI: 10.1038/nature15377
Dynamic m(6)A mRNA methylation directs translational control of heat shock response
Abstract
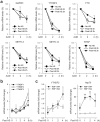
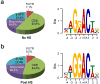
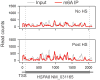
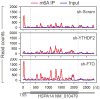
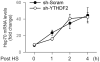
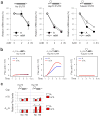
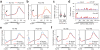
The most abundant mRNA post-transcriptional modification is N(6)-methyladenosine (m(6)A), which has broad roles in RNA biology. In mammalian cells, the asymmetric distribution of m(6)A along mRNAs results in relatively less methylation in the 5' untranslated region (5'UTR) compared to other regions. However, whether and how 5'UTR methylation is regulated is poorly understood. Despite the crucial role of the 5'UTR in translation initiation, very little is known about whether m(6)A modification influences mRNA translation. Here we show that in response to heat shock stress, certain adenosines within the 5'UTR of newly transcribed mRNAs are preferentially methylated. We find that the dynamic 5'UTR methylation is a result of stress-induced nuclear localization of YTHDF2, a well-characterized m(6)A 'reader'. Upon heat shock stress, the nuclear YTHDF2 preserves 5'UTR methylation of stress-induced transcripts by limiting the m(6)A 'eraser' FTO from demethylation. Remarkably, the increased 5'UTR methylation in the form of m(6)A promotes cap-independent translation initiation, providing a mechanism for selective mRNA translation under heat shock stress. Using Hsp70 mRNA as an example, we demonstrate that a single m(6)A modification site in the 5'UTR enables translation initiation independent of the 5' end N(7)-methylguanosine cap. The elucidation of the dynamic features of 5'UTR methylation and its critical role in cap-independent translation not only expands the breadth of physiological roles of m(6)A, but also uncovers a previously unappreciated translational control mechanism in heat shock response.
Figures


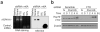




Similar articles
-
Efficient translation initiation directed by the 900-nucleotide-long and GC-rich 5' untranslated region of the human retrotransposon LINE-1 mRNA is strictly cap dependent rather than internal ribosome entry site mediated.Mol Cell Biol. 2007 Jul;27(13):4685-97. doi: 10.1128/MCB.02138-06. Epub 2007 Apr 30. Mol Cell Biol. 2007. PMID: 17470553 Free PMC article.
-
5' UTR m(6)A Promotes Cap-Independent Translation.Cell. 2015 Nov 5;163(4):999-1010. doi: 10.1016/j.cell.2015.10.012. Epub 2015 Oct 22. Cell. 2015. PMID: 26593424 Free PMC article.
-
Migration of Small Ribosomal Subunits on the 5' Untranslated Regions of Capped Messenger RNA.Int J Mol Sci. 2019 Sep 10;20(18):4464. doi: 10.3390/ijms20184464. Int J Mol Sci. 2019. PMID: 31510048 Free PMC article.
-
More than just scanning: the importance of cap-independent mRNA translation initiation for cellular stress response and cancer.Cell Mol Life Sci. 2017 May;74(9):1659-1680. doi: 10.1007/s00018-016-2428-2. Epub 2016 Dec 2. Cell Mol Life Sci. 2017. PMID: 27913822 Free PMC article. Review.
-
Control of translation initiation in animals.Annu Rev Cell Dev Biol. 1998;14:399-458. doi: 10.1146/annurev.cellbio.14.1.399. Annu Rev Cell Dev Biol. 1998. PMID: 9891789 Review.
Cited by
-
RNA Binding by the m6A Methyltransferases METTL16 and METTL3.Biology (Basel). 2024 May 29;13(6):391. doi: 10.3390/biology13060391. Biology (Basel). 2024. PMID: 38927271 Free PMC article. Review.
-
Integrated analysis of the transcriptome-wide m6A methylome in preeclampsia and healthy control placentas.PeerJ. 2020 Sep 15;8:e9880. doi: 10.7717/peerj.9880. eCollection 2020. PeerJ. 2020. PMID: 32983644 Free PMC article.
-
m(6)A: Signaling for mRNA splicing.RNA Biol. 2016 Sep;13(9):756-9. doi: 10.1080/15476286.2016.1201628. Epub 2016 Jun 28. RNA Biol. 2016. PMID: 27351695 Free PMC article. Review.
-
N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression.Elife. 2016 Jul 2;5:e15528. doi: 10.7554/eLife.15528. Elife. 2016. PMID: 27371828 Free PMC article.
-
On the Way to Understanding the Interplay between the RNA Structure and Functions in Cells: A Genome-Wide Perspective.Int J Mol Sci. 2020 Sep 15;21(18):6770. doi: 10.3390/ijms21186770. Int J Mol Sci. 2020. PMID: 32942713 Free PMC article. Review.
References
-
- Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15:293–306. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

