Disulfide Sensitivity in the Env Protein Underlies Lytic Inactivation of HIV-1 by Peptide Triazole Thiols
- PMID: 26458166
- PMCID: PMC4836388
- DOI: 10.1021/acschembio.5b00381
Disulfide Sensitivity in the Env Protein Underlies Lytic Inactivation of HIV-1 by Peptide Triazole Thiols
Abstract
We investigated the mode of action underlying lytic inactivation of HIV-1 virions by peptide triazole thiol (PTT), in particular the relationship between gp120 disulfides and the C-terminal cysteine-SH required for virolysis. Obligate PTT dimer obtained by PTT SH cross-linking and PTTs with serially truncated linkers between pharmacophore isoleucine-ferrocenyltriazole-proline-tryptophan and cysteine-SH were synthesized. PTT variants showed loss of lytic activity but not binding and infection inhibition upon SH blockade. A disproportionate loss of lysis activity vs binding and infection inhibition was observed upon linker truncation. Molecular docking of PTT onto gp120 argued that, with sufficient linker length, the peptide SH could approach and disrupt several alternative gp120 disulfides. Inhibition of lysis by gp120 mAb 2G12, which binds at the base of the V3 loop, as well as disulfide mutational effects, argued that PTT-induced disruption of the gp120 disulfide cluster at the base of the V3 loop is an important step in lytic inactivation of HIV-1. Further, PTT-induced lysis was enhanced after treating virus with reducing agents dithiothreitol and tris (2-carboxyethyl)phosphine. Overall, the results are consistent with the view that the binding of PTT positions the peptide SH group to interfere with conserved disulfides clustered proximal to the CD4 binding site in gp120, leading to disulfide exchange in gp120 and possibly gp41, rearrangement of the Env spike, and ultimately disruption of the viral membrane. The dependence of lysis activity on thiol-disulfide interaction may be related to intrinsic disulfide exchange susceptibility in gp120 that has been reported previously to play a role in HIV-1 cell infection.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



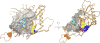


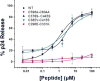
References
-
- Gopi HN, Tirupula KC, Baxter S, Ajith S, Chaiken IM. Click Chemistry on Azidoproline: High-Affinity Dual Antagonist for HIV-1 Envelope Glycoprotein gp120. ChemMedChem. 2006;1:54–57. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

