Nephron organoids derived from human pluripotent stem cells model kidney development and injury
- PMID: 26458176
- PMCID: PMC4747858
- DOI: 10.1038/nbt.3392
Nephron organoids derived from human pluripotent stem cells model kidney development and injury
Abstract
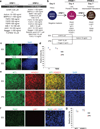
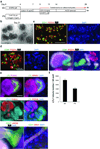
Kidney cells and tissues derived from human pluripotent stem cells (hPSCs) may enable organ regeneration, disease modeling and drug screening. We report an efficient, chemically defined protocol for differentiating hPSCs into multipotent nephron progenitor cells (NPCs) that can form nephron-like structures. By recapitulating metanephric kidney development in vitro, we generate SIX2+ SALL1+ WT1+ PAX2+ NPCs with 90% efficiency within 9 days of differentiation. The NPCs possess the developmental potential of their in vivo counterparts and form PAX8+ LHX1+ renal vesicles that self-organize into nephron structures. In both two- and three-dimensional culture, NPCs form kidney organoids containing epithelial nephron-like structures expressing markers of podocytes, proximal tubules, loops of Henle and distal tubules in an organized, continuous arrangement that resembles the nephron in vivo. We also show that this organoid culture system can be used to study mechanisms of human kidney development and toxicity.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

