Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion
- PMID: 26458510
- PMCID: PMC4602187
- DOI: 10.1038/srep14748
Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion
Abstract
Tumor cell invasion requires the molecular and physical adaptation of both the cell and its microenvironment. Here we show that tumor cells are able to switch between the use of microvesicles and invadopodia to facilitate invasion through the extracellular matrix. Invadopodia formation accompanies the mesenchymal mode of migration on firm matrices and is facilitated by Rac1 activation. On the other hand, during invasion through compliant and deformable environments, tumor cells adopt an amoeboid phenotype and release microvesicles. Notably, firm matrices do not support microvesicle release, whereas compliant matrices are not conducive to invadopodia biogenesis. Furthermore, Rac1 activation is required for invadopodia function, while its inactivation promotes RhoA activation and actomyosin contractility required for microvesicle shedding. Suppression of RhoA signaling blocks microvesicle formation but enhances the formation of invadopodia. Finally, we describe Rho-mediated pathways involved in microvesicle biogenesis through the regulation of myosin light chain phosphatase. Our findings suggest that the ability of tumor cells to switch between the aforementioned qualitatively distinct modes of invasion may allow for dissemination across different microenvironments.
Figures
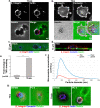
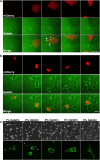




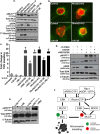
Similar articles
-
ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion.Mol Biol Cell. 2014 Jan;25(1):17-29. doi: 10.1091/mbc.E13-06-0335. Epub 2013 Nov 6. Mol Biol Cell. 2014. PMID: 24196838 Free PMC article.
-
ROCK signaling mediates the adoption of different modes of migration and invasion in human mammary epithelial tumor cells.Exp Cell Res. 2006 Nov 15;312(19):3857-71. doi: 10.1016/j.yexcr.2006.08.025. Epub 2006 Sep 3. Exp Cell Res. 2006. PMID: 17010335
-
MT4-MMP promotes invadopodia formation and cell motility in FaDu head and neck cancer cells.Biochem Biophys Res Commun. 2020 Feb 19;522(4):1009-1014. doi: 10.1016/j.bbrc.2019.12.009. Epub 2019 Dec 5. Biochem Biophys Res Commun. 2020. PMID: 31813546
-
Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis.Trends Cell Biol. 2017 Aug;27(8):595-607. doi: 10.1016/j.tcb.2017.03.003. Epub 2017 Apr 12. Trends Cell Biol. 2017. PMID: 28412099 Free PMC article. Review.
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
Cited by
-
Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment.Annu Rev Pathol. 2023 Jan 24;18:205-229. doi: 10.1146/annurev-pathmechdis-031521-022116. Epub 2022 Oct 6. Annu Rev Pathol. 2023. PMID: 36202098 Free PMC article. Review.
-
Expression of human papillomavirus oncoproteins E6 and E7 inhibits invadopodia activity but promotes cell migration in HPV-positive head and neck squamous cell carcinoma cells.Cancer Rep (Hoboken). 2018 Oct;1(3):e1125. doi: 10.1002/cnr2.1125. Epub 2018 Jul 27. Cancer Rep (Hoboken). 2018. PMID: 32721084 Free PMC article.
-
Plasticity of cancer cell invasion: Patterns and mechanisms.Transl Oncol. 2021 Jan;14(1):100899. doi: 10.1016/j.tranon.2020.100899. Epub 2020 Oct 17. Transl Oncol. 2021. PMID: 33080522 Free PMC article. Review.
-
A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells.Cancers (Basel). 2020 Jan 27;12(2):298. doi: 10.3390/cancers12020298. Cancers (Basel). 2020. PMID: 32012717 Free PMC article. Review.
-
Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers?Front Pharmacol. 2020 Jan 20;10:1500. doi: 10.3389/fphar.2019.01500. eCollection 2019. Front Pharmacol. 2020. PMID: 32038230 Free PMC article. Review.
References
-
- Nguyen D. X., Bos P. D. & Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Can 9, 274–284 (2009). - PubMed
-
- Chaffer C. L. & Weinberg R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011). - PubMed
-
- Spano D., Heck C., De Antonellis P., Christofori G. & Zollo M. Molecular networks that regulate cancer metastasis. Semin Can Biol 22, 234–249 (2012). - PubMed
-
- Yilmaz M. & Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast Rev 28, 15–33 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

