[Ni(cod)2][Al(OR(F))4], a Source for Naked Nickel(I) Chemistry
- PMID: 26458726
- PMCID: PMC5531756
- DOI: 10.1002/anie.201506475
[Ni(cod)2][Al(OR(F))4], a Source for Naked Nickel(I) Chemistry
Abstract
The straightforward synthesis of the cationic, purely organometallic Ni(I) salt [Ni(cod)2](+)[Al(OR(F))4](-) was realized through a reaction between [Ni(cod)2] and Ag[Al(OR(F))4] (cod = 1,5-cyclooctadiene). Crystal-structure analysis and EPR, XANES, and cyclic voltammetry studies confirmed the presence of a homoleptic Ni(I) olefin complex. Weak interactions between the metal center, the ligands, and the anion provide a good starting material for further cationic Ni(I) complexes.
Keywords: crystallography; cyclic voltammetry; density functional calculations; electron paramagnetic resonance; nickel(I) complexes.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

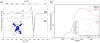
References
-
- Jolly PW, Wilke G. The Organic Chemistry of Nickel. Vol. 2. Academic Press; New York: 1975.
- Jolly PW, Wilke G. The Organic Chemistry of Nickel. Vol. 1. Academic Press; New York: 1974.
- Wilke G. Angew Chem Int Ed Engl. 1988;27:185–206.
- Wilke G. Angew Chem. 1988;100:189–211.
-
- Keim W. Angew Chem. 1990;102:251–260.
-
- Nag K, Chakravorty A. Coord Chem Rev. 1980;33:87–147.
- Wilkinson G, Gillard RD, McCleverty JA. Late transition elements. Pergamon Press; Oxford: 1987. etc.
- Tomlinson Anthony AG. Coord Chem Rev. 1981;37:221–296.
- Schröder M. Coord Chem Rev. 1986;71:139–234.
- Foulds G. Coord Chem Rev. 987;180:1–129.
- Chaloner PA. J Organomet Chem. 1992;432:387–830.
- Chaloner PA. J Organomet Chem. 1992;442:271–519.
- Meyer F, Kozlowski H. Comprehensive Coordination Chemistry 5II6. (Hrsg.: J. A. Meyer, T. J. McCleverty), Pergamon; Oxford: 2003.
- Mitra R, Pörschke KR. Angew Chem Int Ed Engl. 2015;54:7488–7490. - PubMed
-
- Dapporto P, Fallani G, Midollini S, Sacconi L. J Chem Soc, Chem Commun. 1972:1161.
- Dapporto P, Fallani G, Sacconi L. Inorg Chem. 1974;13:2847–2850.
- Dapporto P, Sacconi L. Inorg Chim Acta. 1980;39:61–66.
- Scott Fred, Krüger Carl, Betz Peter. J Organomet Chem. 1990;387:113–121.
- Kitiachvili KD, Mindiola DJ, Hillhouse GL. J Am Chem Soc. 2004;126:10554–10555. - PubMed
- Bradley DC, Hursthouse MB, Smallwood RJ, Welch AJ. J Chem Soc, Chem Commun. 1972:872–873.
- Bai GC, Wei PR, Stephan DW. Organometallics. 2005;24:5901–5908.
- Gleizes A, Dartiguenave M, Dartiguenave Y, Galy J, Klein HF. J Am Chem Soc. 1977;99:5187–5189.
-
- Sacconi L, Dapporto P, Stoppioni P. J Am Chem Soc. 1975;97:5595–5596.
- Sacconi L, Dapporto P, Stoppioni P. Inorg Chem. 1977;16:224–227.
- Furenlid LR, Renner MW, Szalda DJ, Fujita E. J Am Chem Soc. 1991;113:883–892.
- Jung HJ, Bang H, Suh MP. Bull Korean Chem Soc. 2001;22:523–526.
- Suh MP, Kim HK, Kim MJ, Oh KY. Inorg Chem. 1992;31:3620–3625.
- Suh MP, Kim SK. Inorg Chem. 1993;32:3562–3564.
- Suh MP, Oh KY, Lee JW, Bae YY. J Am Chem Soc. 1996;118:777–783.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

