Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial
- PMID: 26458907
- PMCID: PMC4603349
- DOI: 10.1186/s13063-015-0969-6
Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial
Abstract
Background: Extremely premature (birth weight < 1250 g) infants are at high risk for acquiring late-onset sepsis and necrotizing enterocolitis, which are associated with significant mortality and morbidity. Own mother's milk contains protective (immune and trophic) biofactors which provide antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory functions, enhance intestinal microbiota, and promote intestinal maturation. Many of these biofactors are most highly concentrated in the milk expressed by mothers of extremely premature infants. However, since extremely premature infants do not receive oral milk feeds until 32 weeks post-conceptional age, they lack the potential benefit provided by milk (biofactor) exposure to oropharyngeal immunocompetent cells, and this deficiency could contribute to late-onset sepsis and necrotizing enterocolitis. Therefore, oropharyngeal administration of own mother's milk may improve the health outcomes of these infants.
Objectives: To compare the effects of oropharyngeal administration of mother's milk to a placebo, for important clinical outcomes, including (1A) reducing the incidence of late-onset sepsis (primary outcome) and (1B) necrotizing enterocolitis and death (secondary outcomes). To identify the biomechanisms responsible for the beneficial effects of oropharyngeal mother's milk for extremely premature infants, including; (2A) enhancement of gastrointestinal (fecal) microbiota (2B) improvement in antioxidant defense maturation or reduction of pro-oxidant status, and (2C) maturation of immunostimulatory effects as measured by changes in urinary lactoferrin.
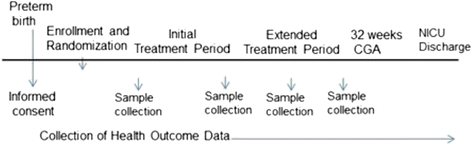
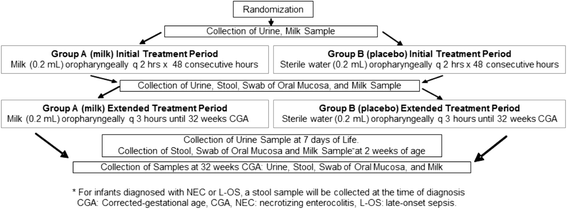
Methods/design: A 5-year, multi-center, double-blind, randomized controlled trial designed to evaluate the safety and efficacy of oropharyngeal mother's milk to reduce the incidence of (1A) late-onset sepsis and (1B) necrotizing enterocolitis and death in a large cohort of extremely premature infants (n = 622; total patients enrolled). Enrolled infants are randomly assigned to one of 2 groups: Group A infants receive 0.2 mL of own mother's milk, via oropharyngeal administration, every 2 hours for 48 hours, then every 3 hours until 32 weeks corrected-gestational age. Group B infants receive a placebo (0.2 mL sterile water) following the same protocol. Milk, urine, oral mucosal swab, and stool samples are collected at various time points, before, during and after the treatment periods. Health outcome and safety data are collected throughout the infant's stay.
Trial registration: ClinicalTrials.gov identifier: NCT02116699 on 11 April 2014. Last updated: 26 May 2015.
Figures
Similar articles
-
Effect of Donor Milk on Severe Infections and Mortality in Very Low-Birth-Weight Infants: The Early Nutrition Study Randomized Clinical Trial.JAMA Pediatr. 2016 Jul 1;170(7):654-61. doi: 10.1001/jamapediatrics.2016.0183. JAMA Pediatr. 2016. PMID: 27135598 Clinical Trial.
-
A randomized controlled trial protocol comparing the feeds of fresh versus frozen mother's own milk for preterm infants in the NICU.Trials. 2020 Feb 11;21(1):170. doi: 10.1186/s13063-019-3981-4. Trials. 2020. PMID: 32046760 Free PMC article.
-
Oropharyngeal administration of mother's milk to prevent necrotizing enterocolitis in extremely low-birth-weight infants: theoretical perspectives.J Perinat Neonatal Nurs. 2015 Jan-Mar;29(1):81-90. doi: 10.1097/JPN.0000000000000087. J Perinat Neonatal Nurs. 2015. PMID: 25633403 Review.
-
Oropharyngeal colostrum administration in extremely premature infants: an RCT.Pediatrics. 2015 Feb;135(2):e357-66. doi: 10.1542/peds.2014-2004. Pediatrics. 2015. PMID: 25624376 Clinical Trial.
-
Oropharyngeal Mother's Milk: State of the Science and Influence on Necrotizing Enterocolitis.Clin Perinatol. 2019 Mar;46(1):77-88. doi: 10.1016/j.clp.2018.09.005. Epub 2018 Dec 13. Clin Perinatol. 2019. PMID: 30771821 Review.
Cited by
-
Oropharyngeal administration of mother's own milk influences levels of salivary sIgA in preterm infants fed by gastric tube.Sci Rep. 2022 Feb 9;12(1):2233. doi: 10.1038/s41598-022-06243-2. Sci Rep. 2022. PMID: 35140309 Free PMC article.
-
Effects of oropharyngeal administration of own mother's milk on oral microbial colonization in very low birth weight infants fed by gastric tube: A randomized controlled trial.Immun Inflamm Dis. 2024 Apr;12(4):e1247. doi: 10.1002/iid3.1247. Immun Inflamm Dis. 2024. PMID: 38629781 Free PMC article. Clinical Trial.
-
Colostrum oropharyngeal immunotherapy for very low birth weight preterm infants: protocol of an intervention study.BMC Pediatr. 2020 Aug 7;20(1):371. doi: 10.1186/s12887-020-02266-8. BMC Pediatr. 2020. PMID: 32767992 Free PMC article. Clinical Trial.
-
Oropharyngeal administration of colostrum for preventing necrotizing enterocolitis and late-onset sepsis in preterm infants with gestational age ≤ 32 weeks: a pilot single-center randomized controlled trial.Int Breastfeed J. 2021 Aug 21;16(1):59. doi: 10.1186/s13006-021-00408-x. Int Breastfeed J. 2021. PMID: 34419090 Free PMC article. Clinical Trial.
-
Low Content of Cyclosporine A and Its Metabolites in the Colostrum of Post-Transplant Mothers.Nutrients. 2020 Sep 4;12(9):2713. doi: 10.3390/nu12092713. Nutrients. 2020. PMID: 32899873 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

