Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia
- PMID: 26459177
- PMCID: PMC4809642
- DOI: 10.1158/1078-0432.CCR-15-0481
Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia
Abstract
Purpose: RG7112 is a small-molecule MDM2 antagonist. MDM2 is a negative regulator of the tumor suppressor p53 and frequently overexpressed in leukemias. Thus, a phase I study of RG7112 in patients with hematologic malignancies was conducted.
Experimental design: Primary study objectives included determination of the dose and safety profile of RG7112. Secondary objectives included evaluation of pharmacokinetics; pharmacodynamics, such as TP53-mutation status and MDM2 expression; and preliminary clinical activity. Patients were divided into two cohorts: Stratum A [relapsed/refractory acute myeloid leukemia (AML; except acute promyelocytic leukemia), acute lymphoblastic leukemia, and chronic myelogenous leukemia] and Stratum B (relapsed/refractory chronic lymphocytic leukemia/small cell lymphocytic leukemia; CLL/sCLL). Some Stratum A patients were treated at the MTD to assess clinical activity.
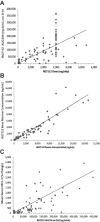
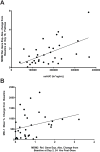
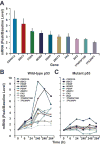
Results: RG7112 was administered to 116 patients (96 patients in Stratum A and 20 patients in Stratum B). All patients experienced at least 1 adverse event, and 3 dose-limiting toxicities were reported. Pharmacokinetic analysis indicated that twice-daily dosing enhanced daily exposure. Antileukemia activity was observed in the 30 patients with AML assessed at the MTD, including 5 patients who met International Working Group (IWG) criteria for response. Exploratory analysis revealed TP53 mutations in 14% of Stratum A patients and in 40% of Stratum B patients. Two patients with TP53 mutations exhibited clinical activity. p53 target genes were induced only in TP53 wild-type leukemic cells. Baseline expression levels of MDM2 correlated positively with clinical response.
Conclusions: RG7112 demonstrated clinical activity against relapsed/refractory AML and CLL/sCLL. MDM2 inhibition resulted in p53 stabilization and transcriptional activation of p53-target genes. We provide proof-of-concept that MDM2 inhibition restores p53 function and generates clinical responses in hematologic malignancies.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. - PubMed
-
- Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17:93–102. - PubMed
-
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45. - PubMed
-
- Chene P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

