Mithramycin Depletes Specificity Protein 1 and Activates p53 to Mediate Senescence and Apoptosis of Malignant Pleural Mesothelioma Cells
- PMID: 26459178
- PMCID: PMC4775437
- DOI: 10.1158/1078-0432.CCR-14-3379
Mithramycin Depletes Specificity Protein 1 and Activates p53 to Mediate Senescence and Apoptosis of Malignant Pleural Mesothelioma Cells
Abstract
Purpose: Specificity protein 1 (SP1) is an oncogenic transcription factor overexpressed in various human malignancies. This study sought to examine SP1 expression in malignant pleural mesotheliomas (MPM) and ascertain the potential efficacy of targeting SP1 in these neoplasms.
Experimental design: qRT-PCR, immunoblotting, and immunohistochemical techniques were used to evaluate SP1 expression in cultured MPM cells and MPM specimens and normal mesothelial cells/pleura. MTS, chemotaxis, soft agar, β-galactosidase, and Apo-BrdUrd techniques were used to assess proliferation, migration, clonogenicity, senescence, and apoptosis in MPM cells following SP1 knockdown, p53 overexpression, or mithramycin treatment. Murine subcutaneous and intraperitoneal xenograft models were used to examine effects of mithramycin on MPM growth in vivo. Microarray, qRT-PCR, immunoblotting, and chromatin immunoprecipitation techniques were used to examine gene expression profiles mediated by mithramycin and combined SP1 knockdown/p53 overexpression and correlate these changes with SP1 and p53 levels within target gene promoters.
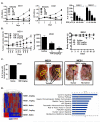
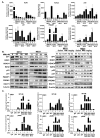
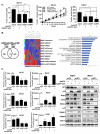
Results: MPM cells and tumors exhibited higher SP1 mRNA and protein levels relative to control cells/tissues. SP1 knockdown significantly inhibited proliferation, migration, and clonogenicity of MPM cells. Mithramycin depleted SP1 and activated p53, dramatically inhibiting proliferation and clonogenicity of MPM cells. Intraperitoneal mithramycin significantly inhibited growth of subcutaneous MPM xenografts and completely eradicated mesothelioma carcinomatosis in 75% of mice. Mithramycin modulated genes mediating oncogene signaling, cell-cycle regulation, senescence, and apoptosis in vitro and in vivo. The growth-inhibitory effects of mithramycin in MPM cells were recapitulated by combined SP1 knockdown/p53 overexpression.
Conclusions: These findings provide preclinical rationale for phase II evaluation of mithramycin in patients with mesothelioma.
©2015 American Association for Cancer Research.
Figures


Similar articles
-
Polycomb repressor complex-2 is a novel target for mesothelioma therapy.Clin Cancer Res. 2012 Jan 1;18(1):77-90. doi: 10.1158/1078-0432.CCR-11-0962. Epub 2011 Oct 25. Clin Cancer Res. 2012. PMID: 22028491 Free PMC article.
-
UHRF1 Is a Novel Druggable Epigenetic Target in Malignant Pleural Mesothelioma.J Thorac Oncol. 2021 Jan;16(1):89-103. doi: 10.1016/j.jtho.2020.08.024. Epub 2020 Sep 11. J Thorac Oncol. 2021. PMID: 32927122 Free PMC article.
-
Functional Analysis of the Adrenomedullin Pathway in Malignant Pleural Mesothelioma.J Thorac Oncol. 2016 Jan;11(1):94-107. doi: 10.1016/j.jtho.2015.09.004. J Thorac Oncol. 2016. PMID: 26762744
-
Molecular Pharmacology of Malignant Pleural Mesothelioma: Challenges and Perspectives From Preclinical and Clinical Studies.Curr Drug Targets. 2016;17(7):824-49. doi: 10.2174/1389450116666150804110714. Curr Drug Targets. 2016. PMID: 26240051 Review.
-
Secreted and Tissue miRNAs as Diagnosis Biomarkers of Malignant Pleural Mesothelioma.Int J Mol Sci. 2018 Feb 17;19(2):595. doi: 10.3390/ijms19020595. Int J Mol Sci. 2018. PMID: 29462963 Free PMC article. Review.
Cited by
-
The Role of Cancer Stem Cells in Colorectal Cancer: From the Basics to Novel Clinical Trials.Cancers (Basel). 2021 Mar 4;13(5):1092. doi: 10.3390/cancers13051092. Cancers (Basel). 2021. PMID: 33806312 Free PMC article. Review.
-
A Bis-Indole-Derived NR4A1 Antagonist Induces PD-L1 Degradation and Enhances Antitumor Immunity.Cancer Res. 2020 Mar 1;80(5):1011-1023. doi: 10.1158/0008-5472.CAN-19-2314. Epub 2020 Jan 7. Cancer Res. 2020. PMID: 31911554 Free PMC article.
-
The Histone Methyltransferase Gene G9A Is Regulated by Nuclear Receptor 4A1 in Alveolar Rhabdomyosarcoma Cells.Mol Cancer Ther. 2021 Mar;20(3):612-622. doi: 10.1158/1535-7163.MCT-20-0474. Epub 2020 Dec 4. Mol Cancer Ther. 2021. PMID: 33277444 Free PMC article.
-
Mithramycin A Enhances Tumor Sensitivity to Mitotic Catastrophe Resulting From DNA Damage.Int J Radiat Oncol Biol Phys. 2018 Feb 1;100(2):344-352. doi: 10.1016/j.ijrobp.2017.09.049. Epub 2017 Oct 12. Int J Radiat Oncol Biol Phys. 2018. PMID: 29157749 Free PMC article.
-
Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma.Int J Nanomedicine. 2017 Jul 24;12:5255-5269. doi: 10.2147/IJN.S139507. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28769562 Free PMC article.
References
-
- International Agency for Research on Cancer . IARC monographs on the evaluation of carcinogenic risks of humans: Volume 100C: a review of human carcinogens: arsenic, metals, fibres, and dust. International Agency for Research on Cancer; Lyon: 2011.
-
- Leuzzi G, Rea F, Spaggiari L, Marulli G, Sperduti I, Alessandrini G, et al. Prognostic score of long-term survival after surgery for malignant pleural mesothelioma: a multicenter analysis. Ann Thorac Surg. 2015;100:890–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

