Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study
- PMID: 26459265
- PMCID: PMC4604076
- DOI: 10.1186/s12916-015-0452-y
Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study
Abstract
Background: The present study aims to evaluate the cost-effectiveness of extending the pre-2013 influenza immunisation programme for high-risk and elderly individuals to those at low risk of developing complications following infection with seasonal influenza.
Methods: We performed an economic evaluation comparing different extensions of the pre-2013 influenza programme to seven possible age groups of low-risk individuals (aged 2-4 years, 50-64 years, 5-16 years, 2-4 and 50-64 years, 2-16 years, 2-16 and 50-64 years, and 2-64 years). These extensions are evaluated incrementally on four base scenarios (no vaccination, risk group only with coverage as observed between 1995 and 2009, risk group and 65+, and risk group with 75% coverage and 65+). Impact of vaccination is assessed using a transmission model built and parameterised from a previously published study. The study population is all individuals of all ages in England and Wales representing an average total of 52.6 million people over 14 influenza seasons (1995-2009).
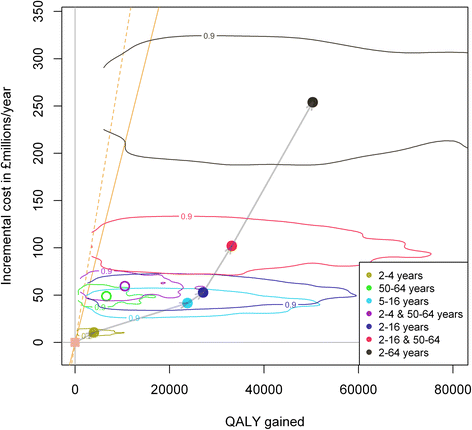
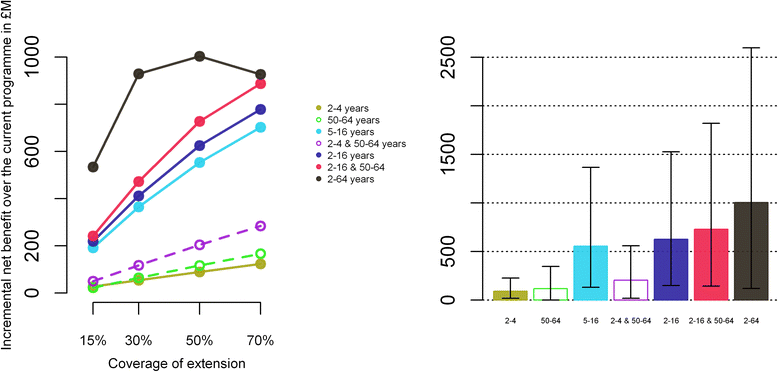
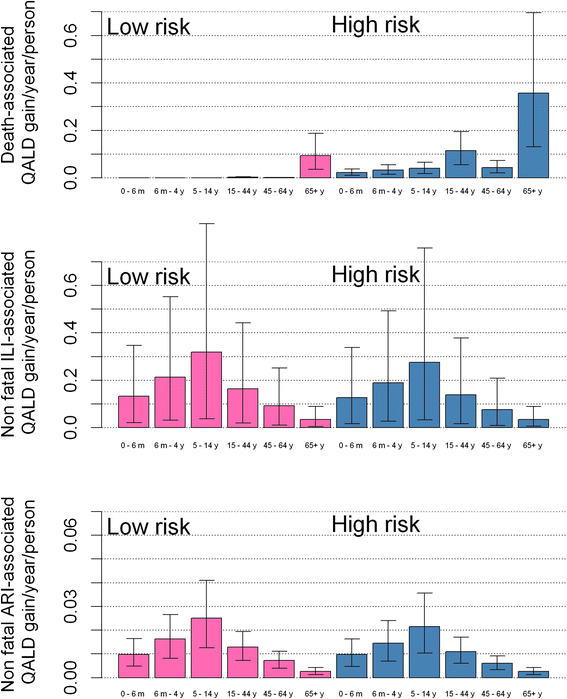
Results: The influenza programme (risk group and elderly) prior to 2013 is likely to be cost effective (incremental cost effectiveness ratio: 7,475 £/QALY, net benefit: 253 M£ [15-829]). Extension to any one of the low-risk target groups defined earlier is likely to be cost-effective. However, strategies that do not include vaccination of school-aged children are less likely to be cost-effective. The most efficient strategy is extension to the 5-16 year age group while universal vaccination (extension to all low-risk individuals over 2 years) will achieve the highest net benefit. While extension to the 2-16 year age group is likely to be very cost effective, the cost-effectiveness of extensions beyond 2-16 years is very uncertain. Extension to the 5-16 year age group would likely remain cost-effective even without herd immunity effects to other age groups. As our study includes a strong historical component, our results depend on the efficacy of the influenza vaccine remaining at levels similar to the ones achieved in the past over a long-period of time (assumed to vary between 28% and 70% depending of the circulating strains and age groups).
Conclusions: Making use of surveillance data from over a decade in conjunction with a dynamic model, we find that vaccination of children in the United Kingdom is likely to be highly cost-effective, not only for their own benefit but also to reduce the disease burden in the rest of the community.
Figures
Similar articles
-
Cost-effectiveness of live-attenuated influenza vaccination among school-age children.Vaccine. 2021 Jan 8;39(2):447-456. doi: 10.1016/j.vaccine.2020.10.007. Epub 2020 Dec 4. Vaccine. 2021. PMID: 33280855
-
Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England.BMC Med. 2017 Sep 8;15(1):166. doi: 10.1186/s12916-017-0932-3. BMC Med. 2017. PMID: 28882149 Free PMC article.
-
Optimising age coverage of seasonal influenza vaccination in England: A mathematical and health economic evaluation.PLoS Comput Biol. 2020 Oct 6;16(10):e1008278. doi: 10.1371/journal.pcbi.1008278. eCollection 2020 Oct. PLoS Comput Biol. 2020. PMID: 33021983 Free PMC article.
-
Implementation of the United Kingdom's childhood influenza national vaccination programme: A review of clinical impact and lessons learned over six influenza seasons.Vaccine. 2020 Aug 10;38(36):5747-5758. doi: 10.1016/j.vaccine.2020.06.065. Epub 2020 Jul 21. Vaccine. 2020. PMID: 32703747 Review.
-
Seasonal influenza vaccines.Curr Top Microbiol Immunol. 2009;333:43-82. doi: 10.1007/978-3-540-92165-3_3. Curr Top Microbiol Immunol. 2009. PMID: 19768400 Review.
Cited by
-
Influenza vaccination: protecting the most vulnerable.Eur Respir Rev. 2021 Jan 13;30(159):200258. doi: 10.1183/16000617.0258-2020. Print 2021 Mar 31. Eur Respir Rev. 2021. PMID: 33650528 Free PMC article. Review.
-
[Health Technology Assessment (HTA) of the introduction of influenza vaccination for Italian children with Fluenz Tetra®].J Prev Med Hyg. 2021 Sep 10;62(2 Suppl 1):E1-E118. doi: 10.15167/2421-4248/jpmh2021.62.2s1. eCollection 2021 Jun. J Prev Med Hyg. 2021. PMID: 34909481 Free PMC article. Italian. No abstract available.
-
Epidemiological drivers of transmissibility and severity of SARS-CoV-2 in England.Nat Commun. 2023 Jul 17;14(1):4279. doi: 10.1038/s41467-023-39661-5. Nat Commun. 2023. PMID: 37460537 Free PMC article.
-
Health and economic impact of seasonal influenza mass vaccination strategies in European settings: A mathematical modelling and cost-effectiveness analysis.Vaccine. 2022 Feb 23;40(9):1306-1315. doi: 10.1016/j.vaccine.2022.01.015. Epub 2022 Jan 31. Vaccine. 2022. PMID: 35109968 Free PMC article.
-
Effect of Pediatric Influenza Vaccination on Antibiotic Resistance, England and Wales.Emerg Infect Dis. 2020 Jan;26(1):138-142. doi: 10.3201/eid2601.191110. Emerg Infect Dis. 2020. PMID: 31574242 Free PMC article.
References
-
- World Health Organisation . Influenza Vaccines – WHO Position Paper. Geneva: WHO; 2005.
-
- Mereckiene J, Cotter S, D’Ancona F, Giambi C, Nicoll A, Lévy-Bruhl D, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008–2009. Eurosurveillance. 2010;15:1–10. - PubMed
-
- World Health Organisation . Weekly epidemiological record. Geneva: WHO; 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

