Sex-Specific Effect of Endothelin in the Blood Pressure Response to Acute Angiotensin II in Growth-Restricted Rats
- PMID: 26459423
- PMCID: PMC4656137
- DOI: 10.1161/HYPERTENSIONAHA.115.06257
Sex-Specific Effect of Endothelin in the Blood Pressure Response to Acute Angiotensin II in Growth-Restricted Rats
Abstract
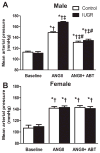
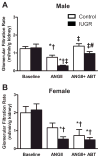
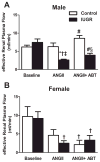
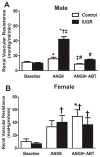
The renal endothelin system contributes to sex differences in blood pressure with males demonstrating greater endothelin type-A receptor-mediated responses relative to females. Intrauterine growth restriction programs hypertension and enhance renal sensitivity to acute angiotensin II in male growth-restricted rats. Endothelin is reported to work synergistically with angiotensin II. Thus, this study tested the hypothesis that endothelin augments the blood pressure response to acute angiotensin II in male growth-restricted rats. Systemic and renal hemodynamics were determined in response to acute angiotensin II (100 mg/kg per minute for 30 minutes) with and without the endothelin type-A receptor antagonist, Atrasentan (ABT-627; 10 ng/kg per minute for 30 minutes), in rats pretreated with enalapril (250 mg/L for 1 week) to normalize the endogenous renin-angiotensin system. Endothelin type-A receptor blockade reduced angiotensin II-mediated increases in blood pressure in male control and male growth-restricted rats. Endothelin type-A receptor blockade also abolished hyper-responsiveness to acute angiotensin II in male growth-restricted rats. Yet, blood pressure remained significantly elevated above baseline after endothelin type-A receptor blockade, suggesting that factors in addition to endothelin contribute to the basic angiotensin II-induced pressor response in male rats. We also determined sex-specific effects of endothelin on acute angiotensin II-mediated hemodynamic responses. Endothelin type-A receptor blockade did not reduce acute angiotensin II-mediated increases in blood pressure in female control or growth-restricted rats, intact or ovariectomized. Thus, these data suggest that endothelin type-A receptor blockade contributes to hypersensitivity to acute angiotensin II in male growth-restricted rats and further supports the sex-specific effect of endothelin on blood pressure.
Keywords: angiotensin II; blood pressure; endothelins; renin–angiotensin system; sex characteristics.
© 2015 American Heart Association, Inc.
Figures
References
-
- Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. - PMC - PubMed
-
- Herrera-Garcia G, Contag S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr Hypertens Rep. 2014;16:1–10. - PubMed
-
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. - PubMed
-
- Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

