Precision of maximum likelihood estimation in adaptive designs
- PMID: 26459506
- PMCID: PMC4755201
- DOI: 10.1002/sim.6761
Precision of maximum likelihood estimation in adaptive designs
Abstract
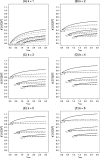
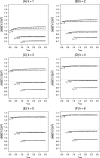
There has been increasing interest in trials that allow for design adaptations like sample size reassessment or treatment selection at an interim analysis. Ignoring the adaptive and multiplicity issues in such designs leads to an inflation of the type 1 error rate, and treatment effect estimates based on the maximum likelihood principle become biased. Whereas the methodological issues concerning hypothesis testing are well understood, it is not clear how to deal with parameter estimation in designs were adaptation rules are not fixed in advanced so that, in practice, the maximum likelihood estimate (MLE) is used. It is therefore important to understand the behavior of the MLE in such designs. The investigation of Bias and mean squared error (MSE) is complicated by the fact that the adaptation rules need not be fully specified in advance and, hence, are usually unknown. To investigate Bias and MSE under such circumstances, we search for the sample size reassessment and selection rules that lead to the maximum Bias or maximum MSE. Generally, this leads to an overestimation of Bias and MSE, which can be reduced by imposing realistic constraints on the rules like, for example, a maximum sample size. We consider designs that start with k treatment groups and a common control and where selection of a single treatment and control is performed at the interim analysis with the possibility to reassess each of the sample sizes. We consider the case of unlimited sample size reassessments as well as several realistically restricted sample size reassessment rules.
Keywords: adaptive designs; maximum likelihood estimation; sample size reassessment; treatment selection.
Copyright © 2015 John Wiley & Sons, Ltd.
Figures


Similar articles
-
Maximum type 1 error rate inflation in multiarmed clinical trials with adaptive interim sample size modifications.Biom J. 2014 Jul;56(4):614-30. doi: 10.1002/bimj.201300153. Epub 2014 Apr 22. Biom J. 2014. PMID: 24753160 Free PMC article.
-
An evaluation of inferential procedures for adaptive clinical trial designs with pre-specified rules for modifying the sample size.Biometrics. 2014 Sep;70(3):556-67. doi: 10.1111/biom.12168. Epub 2014 Apr 25. Biometrics. 2014. PMID: 24766094
-
Estimation in flexible two stage designs.Stat Med. 2006 Oct 15;25(19):3366-81. doi: 10.1002/sim.2258. Stat Med. 2006. PMID: 16220489
-
Methods for flexible sample-size design in clinical trials: Likelihood, weighted, dual test, and promising zone approaches.Contemp Clin Trials. 2016 Mar;47:40-8. doi: 10.1016/j.cct.2015.12.007. Epub 2015 Dec 7. Contemp Clin Trials. 2016. PMID: 26674739 Review.
-
Adaptive designs at European Organisation for Research and Treatment of Cancer (EORTC) with a focus on adaptive sample size re-estimation based on interim-effect size.Eur J Cancer. 2012 Jun;48(9):1386-91. doi: 10.1016/j.ejca.2011.12.024. Epub 2012 Jan 24. Eur J Cancer. 2012. PMID: 22281098 Review.
Cited by
-
Asymptotic properties of maximum likelihood estimators with sample size recalculation.Stat Pap (Berl). 2019 Apr;60(2):373-394. doi: 10.1007/s00362-019-01095-x. Epub 2019 Feb 28. Stat Pap (Berl). 2019. PMID: 31827313 Free PMC article.
-
Distribution Theory Following Blinded and Unblinded Sample Size Re-estimation under Parametric Models.Commun Stat Simul Comput. 2022;51(4):2053-2064. doi: 10.1080/03610918.2019.1692030. Epub 2019 Nov 22. Commun Stat Simul Comput. 2022. PMID: 35755487 Free PMC article.
-
Point estimation for adaptive trial designs II: Practical considerations and guidance.Stat Med. 2023 Jun 30;42(14):2496-2520. doi: 10.1002/sim.9734. Epub 2023 Apr 5. Stat Med. 2023. PMID: 37021359 Free PMC article. Review.
-
Bias induced by adaptive dose-finding designs.J Appl Stat. 2019 Aug 1;47(13-15):2431-2442. doi: 10.1080/02664763.2019.1649375. eCollection 2020. J Appl Stat. 2019. PMID: 35707439 Free PMC article.
References
-
- Proschan MA, Hunsberger SA. Designed extension of studies based on conditional power. Biometrics 1995; 51:1315–1324. - PubMed
-
- Dunnett C. A multiple comparison procedure for comparing several treatments with a control. JASA 1955; 50:1096–1121.
-
- Bauer P, Koehne K. Evaluations of experiments with adaptive interim analysis. Biometrics 1994; 50:1029–1041. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
