VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption
- PMID: 26459520
- PMCID: PMC4644375
- DOI: 10.15252/emmm.201505731
VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption
Abstract
Vascular endothelial growth factor C (VEGF-C) binding to its tyrosine kinase receptor VEGFR-3 drives lymphatic vessel growth during development and in pathological processes. Although the VEGF-C/VEGFR-3 pathway provides a target for treatment of cancer and lymphedema, the physiological functions of VEGF-C in adult vasculature are unknown. We show here that VEGF-C is necessary for perinatal lymphangiogenesis, but required for adult lymphatic vessel maintenance only in the intestine. Following Vegfc gene deletion in adult mice, the intestinal lymphatic vessels, including the lacteal vessels, underwent gradual atrophy, which was aggravated when also Vegfd was deleted. VEGF-C was expressed by a subset of smooth muscle cells adjacent to the lacteals in the villus and in the intestinal wall. The Vegfc-deleted mice showed defective lipid absorption and increased fecal excretion of dietary cholesterol and fatty acids. When fed a high-fat diet, the Vegfc-deficient mice were resistant to obesity and had improved glucose metabolism. Our findings indicate that the lymphangiogenic growth factors provide trophic and dynamic regulation of the intestinal lymphatic vasculature, which could be especially important in the dietary regulation of adiposity and cholesterol metabolism.
Keywords: VEGF‐C; cholesterol; lipid absorption; lymphatic vasculature; obesity.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
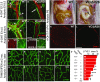
A–D Mesenteric blood (PECAM1, red) and lymphatic vessels (LYVE1, green; PROX1, gray). Asterisks indicate lymphatic valves, arrow indicates a lymphatic vessel stub, and arrowhead indicates an isolated lymphatic vessel fragment
E–H Blood vessels (PECAM1, green) and lymphatic vessels (VEGFR-3, red) in the small intestinal wall.
I, J Detection of chylous ascites (arrows) in the VCiΔR26 mice at P6.
K, L LYVE1 staining of intestinal lymphatic vessels at P6.
M–T LYVE1 staining of lymphatic vessels in the intestinal wall in adult mice. Genotypes and deletion lengths are indicated in (U).
U Quantification of LYVE1 areas in (M–T). Length of the i∆Vegfc gene deletion is indicated in months (mo), and Vegfd indicates the VEGF-D genotype. Data are represented as mean ± SEM. Significant differences were determined using one-way ANOVA and Bonferroni post hoc analysis compared to WT intestine represented in (M). *P = 0.003, **P = 0.001, ***P = 0.0008, ♯P = 0.0002, §P = 0.0001.
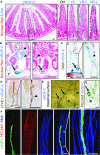
Overview of the small intestine cross section stained with nuclear red and highlighting the location of higher magnification images in (B–F); (I) for the entire villus and (II) for the villus base. β-Gal staining pattern of the villus in wild-type (Ctrl), Vegfc/LacZ (VC), Vegfr3/LacZ (VR-3), and Vegfr2/LacZ (VR-2) mice.
Higher magnification images representing β-Gal staining of the villus base in Vegfc/LacZ mice.
Vegfc/LacZ β-Gal staining reaction with smooth muscle actin (SMA) peroxidase staining.
Vegfc/LacZ β-Gal staining and LYVE1 peroxidase staining.
Surface image of Vegfc/LacZ β-Gal-stained intestine (left) and cross section counterstaining with PECAM1 (right).
Immunofluorescence staining of lacteal lymphatic vessel (LYVE1), blood capillaries (PECAM1), and smooth muscle cells (SMA).
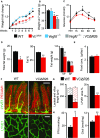
Body weight change during seven weeks of HFD, expressed as average fold change in comparison with the starting weight. n = 16, WT; n = 6, Vegfd−/−; n = 16, VCiΔR26; n = 5, Vegfd−/−; VCiΔR26.
Body weight comparisons at seven weeks of HFD. Significant differences were determined using one-way ANOVA and Bonferroni post hoc analysis compared to WT. *P = 0.004; **P = 0.003. n = 16, WT; n = 6, Vegfd−/−; n = 16, VCiΔR26; n = 5, Vegfd−/−; VCiΔR26.
Glucose tolerance test (GTT) after six weeks of HFD. Significant differences were determined using unpaired two-tailed t-test. *P = 0.014; **P = 0.041. n = 5, WT; n = 6, VCiΔR26.
Total fat weight, fat percentage from body composition measurements after six weeks of HFD, and weights of visceral fat (VF) and subcutaneous fat (SF) at the time of necropsy. Significant differences were determined using unpaired two-tailed t-test. *P = 0.006; **P = 0.001; #P = 0.008; §P = 0.006. n = 4 in each group.
Food consumption during the fifth week of HFD. n = 9, WT; n = 10, VCiΔR26.
Whole-mount immunofluorescence staining of blood (PECAM1, red) and lymphatic vessels (LYVE1, green) in intestinal villi and intestinal wall.
Quantification of the lacteal and villus length (solid and striped color bars, respectively) and the intestinal wall LYVE1+ area percentage from images represented in (F). Significant differences were determined using unpaired two-tailed t-test. *P = 0.0002; **P = 0.00007. n = 5, WT; n = 6, VCiΔR26.
Free fatty acid (FFA) and cholesterol measurements from the feces after six weeks of HFD. Significant differences were determined using unpaired two-tailed t-test. *P = 0.001; **P = 0.007. n = 5, WT; n = 6, VCiΔR26.
References
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. - PubMed
-
- Astin JW, Haggerty MJ, Okuda KS, Le Guen L, Misa JP, Tromp A, Hogan BM, Crosier KE, Crosier PS. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development. 2014;141:2680–2690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

