Inhibition of β-catenin-TCF1 interaction delays differentiation of mouse embryonic stem cells
- PMID: 26459597
- PMCID: PMC4602028
- DOI: 10.1083/jcb.201503017
Inhibition of β-catenin-TCF1 interaction delays differentiation of mouse embryonic stem cells
Abstract
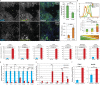
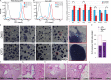
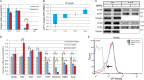
The ability of mouse embryonic stem cells (mESCs) to self-renew or differentiate into various cell lineages is regulated by signaling pathways and a core pluripotency transcriptional network (PTN) comprising Nanog, Oct4, and Sox2. The Wnt/β-catenin pathway promotes pluripotency by alleviating T cell factor TCF3-mediated repression of the PTN. However, it has remained unclear how β-catenin's function as a transcriptional activator with TCF1 influences mESC fate. Here, we show that TCF1-mediated transcription is up-regulated in differentiating mESCs and that chemical inhibition of β-catenin/TCF1 interaction improves long-term self-renewal and enhances functional pluripotency. Genetic loss of TCF1 inhibited differentiation by delaying exit from pluripotency and conferred a transcriptional profile strikingly reminiscent of self-renewing mESCs with high Nanog expression. Together, our data suggest that β-catenin's function in regulating mESCs is highly context specific and that its interaction with TCF1 promotes differentiation, further highlighting the need for understanding how its individual protein-protein interactions drive stem cell fate.
© 2015 Chatterjee et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

