Ribosome binding induces repositioning of the signal recognition particle receptor on the translocon
- PMID: 26459600
- PMCID: PMC4602035
- DOI: 10.1083/jcb.201502103
Ribosome binding induces repositioning of the signal recognition particle receptor on the translocon
Abstract
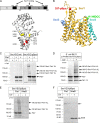
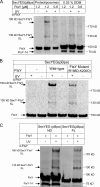
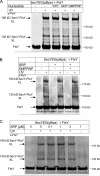
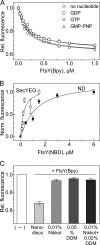
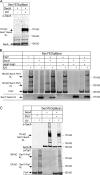
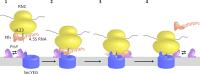
Cotranslational protein targeting delivers proteins to the bacterial cytoplasmic membrane or to the eukaryotic endoplasmic reticulum membrane. The signal recognition particle (SRP) binds to signal sequences emerging from the ribosomal tunnel and targets the ribosome-nascent-chain complex (RNC) to the SRP receptor, termed FtsY in bacteria. FtsY interacts with the fifth cytosolic loop of SecY in the SecYEG translocon, but the functional role of the interaction is unclear. By using photo-cross-linking and fluorescence resonance energy transfer measurements, we show that FtsY-SecY complex formation is guanosine triphosphate independent but requires a phospholipid environment. Binding of an SRP-RNC complex exposing a hydrophobic transmembrane segment induces a rearrangement of the SecY-FtsY complex, which allows the subsequent contact between SecY and ribosomal protein uL23. These results suggest that direct RNC transfer to the translocon is guided by the interaction between SRP and translocon-bound FtsY in a quaternary targeting complex.
© 2015 Kuhn et al.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

