APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments
- PMID: 26459602
- PMCID: PMC4602042
- DOI: 10.1083/jcb.201311117
APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments
Abstract
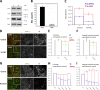
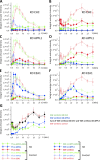
Endocytosis allows cargo to enter a series of specialized endosomal compartments, beginning with early endosomes harboring Rab5 and its effector EEA1. There are, however, additional structures labeled by the Rab5 effector APPL1 whose role in endocytic transport remains unclear. It has been proposed that APPL1 vesicles are transport intermediates that convert into EEA1 endosomes. Here, we tested this model by analyzing the ultrastructural morphology, kinetics of cargo transport, and stability of the APPL1 compartment over time. We found that APPL1 resides on a tubulo-vesicular compartment that is capable of sorting cargo for recycling or degradation and that displays long lifetimes, all features typical of early endosomes. Fitting mathematical models to experimental data rules out maturation of APPL1 vesicles into EEA1 endosomes as a primary mechanism for cargo transport. Our data suggest instead that APPL1 endosomes represent a distinct population of Rab5-positive sorting endosomes, thus providing important insights into the compartmental organization of the early endocytic pathway.
© 2015 Kalaidzidis et al.
Figures






References
-
- Cheng K.K., Lam K.S., Wu D., Wang Y., Sweeney G., Hoo R.L., Zhang J., and Xu A.. 2012. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc. Natl. Acad. Sci. USA. 109:8919–8924. 10.1073/pnas.1202435109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

