Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model
- PMID: 26459826
- PMCID: PMC5410110
- DOI: 10.1099/jgv.0.000309
Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model
Abstract
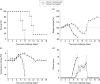
Ebola virus (EBOV) is highly pathogenic, with a predisposition to cause outbreaks in human populations accompanied by significant mortality. Owing to the lack of approved therapies, screening programmes of potentially efficacious drugs have been undertaken. One of these studies has demonstrated the possible utility of chloroquine against EBOV using pseudotyped assays. In mouse models of EBOV disease there are conflicting reports of the therapeutic effects of chloroquine. There are currently no reports of its efficacy using the larger and more stringent guinea pig model of infection. In this study we have shown that replication of live EBOV is impaired by chloroquine in vitro. However, no protective effects were observed in vivo when EBOV-infected guinea pigs were treated with chloroquine. These results advocate that chloroquine should not be considered as a treatment strategy for EBOV.
Figures


References
-
- Center for Drug Evaluation and Research (2005). Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers Washington, DC: US Department of Health and Human Services; .
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

