Cyanobacterial Alkanes Modulate Photosynthetic Cyclic Electron Flow to Assist Growth under Cold Stress
- PMID: 26459862
- PMCID: PMC4602277
- DOI: 10.1038/srep14894
Cyanobacterial Alkanes Modulate Photosynthetic Cyclic Electron Flow to Assist Growth under Cold Stress
Abstract
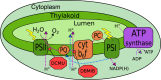
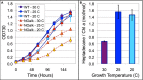
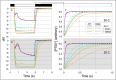
All cyanobacterial membranes contain diesel-range C15-C19 hydrocarbons at concentrations similar to chlorophyll. Recently, two universal but mutually exclusive hydrocarbon production pathways in cyanobacteria were discovered. We engineered a mutant of Synechocystis sp. PCC 6803 that produces no alkanes, which grew poorly at low temperatures. We analyzed this defect by assessing the redox kinetics of PSI. The mutant exhibited enhanced cyclic electron flow (CEF), especially at low temperature. CEF raises the ATP:NADPH ratio from photosynthesis and balances reductant requirements of biosynthesis with maintaining the redox poise of the electron transport chain. We conducted in silico flux balance analysis and showed that growth rate reaches a distinct maximum for an intermediate value of CEF equivalent to recycling 1 electron in 4 from PSI to the plastoquinone pool. Based on this analysis, we conclude that the lack of membrane alkanes causes higher CEF, perhaps for maintenance of redox poise. In turn, increased CEF reduces growth by forcing the cell to use less energy-efficient pathways, lowering the quantum efficiency of photosynthesis. This study highlights the unique and universal role of medium-chain hydrocarbons in cyanobacterial thylakoid membranes: they regulate redox balance and reductant partitioning in these oxygenic photosynthetic cells under stress.
Figures




Similar articles
-
Cold stress effects on PSI photochemistry in Zea mays: differential increase of FQR-dependent cyclic electron flow and functional implications.Plant Cell Physiol. 2011 Jun;52(6):1042-54. doi: 10.1093/pcp/pcr056. Epub 2011 May 4. Plant Cell Physiol. 2011. PMID: 21546369
-
A strain of Synechocystis sp. PCC 6803 without photosynthetic oxygen evolution and respiratory oxygen consumption: implications for the study of cyclic photosynthetic electron transport.Planta. 2001 Nov;214(1):46-56. doi: 10.1007/s004250100578. Planta. 2001. PMID: 11762170
-
Photoheterotrophic fluxome in Synechocystis sp. strain PCC 6803 and its implications for cyanobacterial bioenergetics.J Bacteriol. 2015 Mar;197(5):943-50. doi: 10.1128/JB.02149-14. Epub 2014 Dec 22. J Bacteriol. 2015. PMID: 25535269 Free PMC article.
-
Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions.Plant Cell Physiol. 2010 Dec;51(12):1951-63. doi: 10.1093/pcp/pcq173. Epub 2010 Nov 10. Plant Cell Physiol. 2010. PMID: 21068108 Review.
-
Physiological Significance of NAD Kinases in Cyanobacteria.Front Plant Sci. 2019 Jun 27;10:847. doi: 10.3389/fpls.2019.00847. eCollection 2019. Front Plant Sci. 2019. PMID: 31316540 Free PMC article. Review.
Cited by
-
Antenna Modification Leads to Enhanced Nitrogenase Activity in a High Light-Tolerant Cyanobacterium.mBio. 2021 Dec 21;12(6):e0340821. doi: 10.1128/mbio.03408-21. Epub 2021 Dec 21. mBio. 2021. PMID: 34933453 Free PMC article.
-
Insights into cyanobacterial alkane biosynthesis.J Ind Microbiol Biotechnol. 2022 Apr 14;49(2):kuab075. doi: 10.1093/jimb/kuab075. J Ind Microbiol Biotechnol. 2022. PMID: 34718648 Free PMC article. Review.
-
Absence of alka(e)nes triggers profound remodeling of glycerolipid and carotenoid composition in cyanobacteria membrane.Plant Physiol. 2024 Sep 2;196(1):397-408. doi: 10.1093/plphys/kiae319. Plant Physiol. 2024. PMID: 38850059 Free PMC article.
-
Hydrocarbons Are Essential for Optimal Cell Size, Division, and Growth of Cyanobacteria.Plant Physiol. 2016 Nov;172(3):1928-1940. doi: 10.1104/pp.16.01205. Epub 2016 Oct 5. Plant Physiol. 2016. PMID: 27707888 Free PMC article.
-
Uncovering the substrate of olefin synthase loading domains in cyanobacteria Picosynechococcus sp. strain PCC 7002.RSC Chem Biol. 2025 Jan 14;6(2):307-316. doi: 10.1039/d4cb00234b. eCollection 2025 Feb 5. RSC Chem Biol. 2025. PMID: 39817101 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

