The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface
- PMID: 26460007
- PMCID: PMC4629323
- DOI: 10.1073/pnas.1517724112
The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface
Abstract
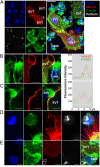
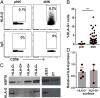
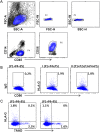
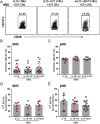
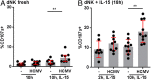
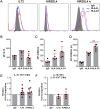
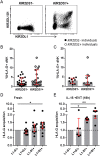
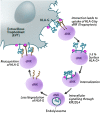
The interaction of noncytotoxic decidual natural killer cells (dNK) and extravillous trophoblasts (EVT) at the maternal-fetal interface was studied. Confocal microscopy revealed that many dNK interact with a single large EVT. Filamentous projections from EVT enriched in HLA-G were shown to contact dNK, and may represent the initial stage of synapse formation. As isolated, 2.5% of dNK contained surface HLA-G. However, surface HLA-G-negative dNK contained internalized HLA-G. Activation of dNK resulted in the disappearance of internalized HLA-G in parallel with restoration of cytotoxicity. Surface HLA-G was reacquired by incubation with EVT. This HLA-G cycle of trogocytosis, endocytosis, degradation, and finally reacquisition provides a transient and localized acquisition of new functional properties by dNK upon interaction with EVT. Interruption of the cycle by activation of dNK by cytokines and/or viral products serves to ensure the NK control of virus infection at the interface, and is illustrated here by the response of dNK to human cytomegalo virus (HCMV)-infected decidual stromal cells. Thus, the HLA-G cycle in dNK can provide both for NK tolerance and antiviral immunity.
Keywords: HCMV; cytotoxicity; decidua; human; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections.J Reprod Immunol. 2017 Feb;119:85-90. doi: 10.1016/j.jri.2016.08.001. Epub 2016 Aug 2. J Reprod Immunol. 2017. PMID: 27523927 Free PMC article. Review.
-
Expression of KIR2DS1 by decidual natural killer cells increases their ability to control placental HCMV infection.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):15072-15077. doi: 10.1073/pnas.1617927114. Epub 2016 Dec 12. Proc Natl Acad Sci U S A. 2016. PMID: 27956621 Free PMC article.
-
Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy.Front Immunol. 2021 May 12;12:663660. doi: 10.3389/fimmu.2021.663660. eCollection 2021. Front Immunol. 2021. PMID: 34054831 Free PMC article. Review.
-
Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7219-24. doi: 10.1073/pnas.1507977112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26015573 Free PMC article.
-
The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy.Cell Mol Immunol. 2016 Jan;13(1):73-81. doi: 10.1038/cmi.2014.126. Epub 2015 Jan 12. Cell Mol Immunol. 2016. PMID: 25578313 Free PMC article.
Cited by
-
HLA-G: A New Immune Checkpoint in Cancer?Int J Mol Sci. 2020 Jun 25;21(12):4528. doi: 10.3390/ijms21124528. Int J Mol Sci. 2020. PMID: 32630545 Free PMC article. Review.
-
Intercellular transfer of HLA-G: its potential in cancer immunology.Clin Transl Immunology. 2019 Aug 30;8(9):e1077. doi: 10.1002/cti2.1077. eCollection 2019. Clin Transl Immunology. 2019. PMID: 31489189 Free PMC article. Review.
-
High-grade serous ovarian tumor cells modulate NK cell function to create an immune-tolerant microenvironment.Cell Rep. 2021 Aug 31;36(9):109632. doi: 10.1016/j.celrep.2021.109632. Cell Rep. 2021. PMID: 34469729 Free PMC article.
-
Soluble human leukocyte antigen -G during pregnancy and infancy in Benin: Mother/child resemblance and association with the risk of malaria infection and low birth weight.PLoS One. 2017 Feb 6;12(2):e0171117. doi: 10.1371/journal.pone.0171117. eCollection 2017. PLoS One. 2017. PMID: 28166246 Free PMC article.
-
Hormonal stimulation reduces numbers and impairs function of human uterine natural killer cells during implantation.Hum Reprod. 2023 Jun 1;38(6):1047-1059. doi: 10.1093/humrep/dead069. Hum Reprod. 2023. PMID: 37075311 Free PMC article.
References
-
- Kovats S, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220–223. - PubMed
-
- Pazmany L, et al. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274(5288):792–795. - PubMed
-
- Poehlmann TG, et al. Inhibition of term decidual NK cell cytotoxicity by soluble HLA-G1. Am J Reprod Immunol. 2006;56(5-6):275–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

