Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease
- PMID: 26460017
- PMCID: PMC4629324
- DOI: 10.1073/pnas.1509914112
Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease
Abstract
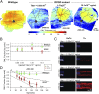
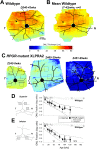
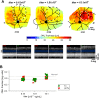
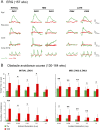
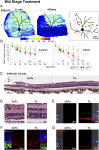
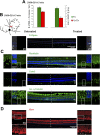
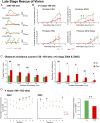
Inherited retinal degenerations cause progressive loss of photoreceptor neurons with eventual blindness. Corrective or neuroprotective gene therapies under development could be delivered at a predegeneration stage to prevent the onset of disease, as well as at intermediate-degeneration stages to slow the rate of progression. Most preclinical gene therapy successes to date have been as predegeneration interventions. In many animal models, as well as in human studies, to date, retinal gene therapy administered well after the onset of degeneration was not able to modify the rate of progression even when successfully reversing dysfunction. We evaluated consequences of gene therapy delivered at intermediate stages of disease in a canine model of X-linked retinitis pigmentosa (XLRP) caused by a mutation in the Retinitis Pigmentosa GTPase Regulator (RPGR) gene. Spatiotemporal natural history of disease was defined and therapeutic dose selected based on predegeneration results. Then interventions were timed at earlier and later phases of intermediate-stage disease, and photoreceptor degeneration monitored with noninvasive imaging, electrophysiological function, and visual behavior for more than 2 y. All parameters showed substantial and significant arrest of the progressive time course of disease with treatment, which resulted in long-term improved retinal function and visual behavior compared with control eyes. Histology confirmed that the human RPGR transgene was stably expressed in photoreceptors and associated with improved structural preservation of rods, cones, and ON bipolar cells together with correction of opsin mislocalization. These findings in a clinically relevant large animal model demonstrate the long-term efficacy of RPGR gene augmentation and substantially broaden the therapeutic window for intervention in patients with RPGR-XLRP.
Keywords: RPGR; XLRP; gene therapy; late stage; retinal degeneration.
Conflict of interest statement
Conflict of interest statement: W.A.B., A.V.C., W.-T.D., S.L.B., A.S.L., W.W.H., S.G.J., and G.D.A. are inventors on the following patent application: PCT/US2013/022628. W.W.H. and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
Figures






References
-
- Wimo A, Prince M. World Alzheimer Report 2010 The Global Ecomomic Impact of Dementia - Executive summary. Alzheimer's Disease International; London: 2010. pp. 1–9.
-
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. CDBE2010 study group European Brain Council The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–162. - PubMed
-
- Li C, Ebrahimi A, Schluesener H. Drug pipeline in neurodegeneration based on transgenic mice models of Alzheimer’s disease. Ageing Res Rev. 2013;12(1):116–140. - PubMed
Publication types
MeSH terms
Grants and funding
- R24EY-022012/EY/NEI NIH HHS/United States
- P30EY-001583/EY/NEI NIH HHS/United States
- R01EY-013203/EY/NEI NIH HHS/United States
- P40 RR002512/RR/NCRR NIH HHS/United States
- P40-OD010939/OD/NIH HHS/United States
- R01 EY006855/EY/NEI NIH HHS/United States
- R01 EY013203/EY/NEI NIH HHS/United States
- R01EY-06855/EY/NEI NIH HHS/United States
- P30 EY001583/EY/NEI NIH HHS/United States
- R24 EY022012/EY/NEI NIH HHS/United States
- P40 OD010939/OD/NIH HHS/United States
- R01 EY017549/EY/NEI NIH HHS/United States
- 2PNEY-018241/PHS HHS/United States
- R01EY-017549/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

