Structural analysis of nucleosomal barrier to transcription
- PMID: 26460019
- PMCID: PMC4629332
- DOI: 10.1073/pnas.1508371112
Structural analysis of nucleosomal barrier to transcription
Abstract
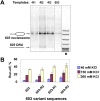
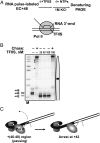
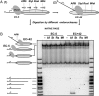
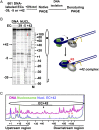
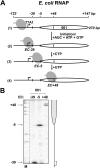
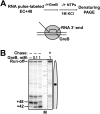
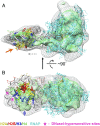
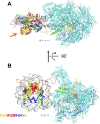
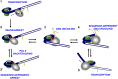
Thousands of human and Drosophila genes are regulated at the level of transcript elongation and nucleosomes are likely targets for this regulation. However, the molecular mechanisms of formation of the nucleosomal barrier to transcribing RNA polymerase II (Pol II) and nucleosome survival during/after transcription remain unknown. Here we show that both DNA-histone interactions and Pol II backtracking contribute to formation of the barrier and that nucleosome survival during transcription likely occurs through allosterically stabilized histone-histone interactions. Structural analysis indicates that after Pol II encounters the barrier, the enzyme backtracks and nucleosomal DNA recoils on the octamer, locking Pol II in the arrested state. DNA is displaced from one of the H2A/H2B dimers that remains associated with the octamer. The data reveal the importance of intranucleosomal DNA-protein and protein-protein interactions during conformational changes in the nucleosome structure on transcription. Mechanisms of nucleosomal barrier formation and nucleosome survival during transcription are proposed.
Keywords: RNA polymerase II; backtracking; chromatin; elongation; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20(3):259–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

