Glycine activated ion channel subunits encoded by ctenophore glutamate receptor genes
- PMID: 26460032
- PMCID: PMC4640758
- DOI: 10.1073/pnas.1513771112
Glycine activated ion channel subunits encoded by ctenophore glutamate receptor genes
Abstract
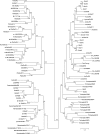
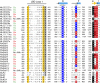
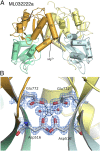
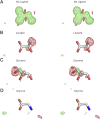
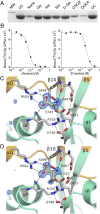
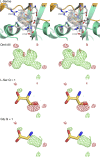
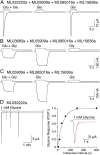
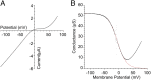
Recent genome projects for ctenophores have revealed the presence of numerous ionotropic glutamate receptors (iGluRs) in Mnemiopsis leidyi and Pleurobrachia bachei, among our earliest metazoan ancestors. Sequence alignments and phylogenetic analysis show that these form a distinct clade from the well-characterized AMPA, kainate, and NMDA iGluR subtypes found in vertebrates. Although annotated as glutamate and kainate receptors, crystal structures of the ML032222a and PbiGluR3 ligand-binding domains (LBDs) reveal endogenous glycine in the binding pocket, whereas ligand-binding assays show that glycine binds with nanomolar affinity; biochemical assays and structural analysis establish that glutamate is occluded from the binding cavity. Further analysis reveals ctenophore-specific features, such as an interdomain Arg-Glu salt bridge, present only in subunits that bind glycine, but also a conserved disulfide in loop 1 of the LBD that is found in all vertebrate NMDA but not AMPA or kainate receptors. We hypothesize that ctenophore iGluRs are related to an early ancestor of NMDA receptors, suggesting a common evolutionary path for ctenophores and bilaterian species, and suggest that future work should consider both glycine and glutamate as candidate neurotransmitters in ctenophore species.
Keywords: NMDA receptors; crystal structures; ctenophores; evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Srivastava M, et al. The Trichoplax genome and the nature of placozoans. Nature. 2008;454(7207):955–960. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

