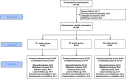
Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: A CANMAT randomized double-blind trial
- PMID: 26460229
- PMCID: PMC4960445
- DOI: 10.1038/mp.2015.158
Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: A CANMAT randomized double-blind trial
Abstract
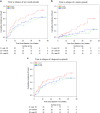
Atypical antipsychotic adjunctive therapy to lithium or valproate is effective in treating acute mania. Although continuation of atypical antipsychotic adjunctive therapy after mania remission reduces relapse of mood episodes, the optimal duration is unknown. As many atypical antipsychotics cause weight gain and metabolic syndrome, they should not be continued unless the benefits outweigh the risks. This 52-week double-blind placebo-controlled trial recruited patients with bipolar I disorder (n=159) who recently remitted from a manic episode during treatment with risperidone or olanzapine adjunctive therapy to lithium or valproate. Patients were randomized to one of three conditions: discontinuation of risperidone or olanzapine and substitution with placebo at (i) entry ('0-weeks' group) or (ii) at 24 weeks after entry ('24-weeks' group) or (iii) continuation of risperidone or olanzapine for the full duration of the study ('52-weeks' group). The primary outcome measure was time to relapse of any mood episode. Compared with the 0-weeks group, the time to any mood episode was significantly longer in the 24-weeks group (hazard ratio (HR) 0.53; 95% confidence interval (CI): 0.33, 0.86) and nearly so in the 52-weeks group (HR: 0.63; 95% CI: 0.39, 1.02). The relapse rate was similar in the 52-weeks group compared with the 24-weeks group (HR: 1.18; 95% CI: 0.71, 1.99); however, sub-group analysis showed discordant results between the two antipsychotics (HR: 0.48, 95% CI: 0.17; 1.32 olanzapine patients; HR: 1.85, 95% CI: 1.00, 3.41 risperidone patients). Average weight gain was 3.2 kg in the 52-weeks group compared with a weight loss of 0.2 kg in the 0-weeks and 0.1 kg in the 24-weeks groups. These findings suggest that risperidone or olanzapine adjunctive therapy for 24 weeks is beneficial but continuation of risperidone beyond this period does not reduce the risk of relapse. Whether continuation of olanzapine beyond this period reduces relapse risk remains unclear but the potential benefit needs to be weighed against an increased risk of weight gain.
Conflict of interest statement
Dr Lakshmi N Yatham reports grants from Lilly, Janssen and from the Canadian Institutes of Health Research during the conduct of the study. He also received grants and personal fees from AstraZeneca, Bristol Myers Squibb and Dianippon Sumitomo. He received personal fees from Forrest, Glaxo Smithkline, Lundbeck, Sunovion and Lilly outside the submitted work. Dr Serge Beaulieu reports grants and personal fees from Astra Zeneca, BMS, Lundbeck, Otsuka and Sunovion. He received grants from Novartis, Canadian Institutes of Health Research, Fonds de recherche du Québec, Réseau Santé Mentale au Québec, Brain and Behavior Research Foundation, Stanley Foundation and Pfizer Reseach Award. He reports receiving personal fees from Forest Laboratories, Lilly, Merck and Pfizer from outside the submitted work. Dr Ayal Schaffer reports grants from Canadian Institute of Health Research during the conduct of the study. He received grants from Pfizer Canada and personal fees from AstraZeneca, Eli Lilly, Sunovion, Lundbeck and Bristol-Myers Squibb outside the submitted work. Dr Marcia Kauer-Sant'Anna reports grants from Stanley Medical Research Institute, grants and personal fees from Eli-Lilly, and grants from CNPq-INCT-TM, CNPq-Universal, FIPE-HCPA and NARSAD outside the submitted work. Dr Flavio Kapzinski reports receiving support for research from AstraZeneca, Eli Lilly, Janssen-Cilag and Servier outside the submitted work. Dr Verinder Sharma reports grant support from Bristol-Myers Squibb, Cephalon, Elan Pharmaceuticals and Shire; personal fees from AstraZeneca, Eli Lilly, Janssen, Servier, Sunovion, Lundbeck and Bristol-Myers Squibb outside the submitted work. Dr Sagar V Parikh reports personal fees from Sunovion, Pfizer, BMS, Otsuka, Astra-Zeneca, Lundbeck and Lilly, and grant funding from Lundbeck outside the submitted work. Dr Andree Daigneault reports grants and personal fees from Astra Zeneca, BMS and personal fees from Lundbeck, Sunovion and Valeant, outside the submitted work. Dr David J Bond reports grants from Pfizer and personal fees from Sunovion, Pfizer, BMS, Otsuka, Astra-Zeneca and Janssen, outside the submitted work. Dr Roumen Milev reports grants and personal fees from Astra Zeneca, BMS, Pfizer and Eli Lilly. He also reports personal fees from Merck, Otsuka, Sunovion, Valeant, as well as personal fees and non-financial support from Lundbeck outside the submitted work. Dr Philippe Baruch reports personal fees from BMS and Sunovion, outside the submitted work. Dr Raymond W. Lam reports grants from Canadian Institutes of Health Research, other from Janssen and Eli Lilly, during the conduct of the study. He also received grants from Bristol Myers Squibb, Coast Capital Savings, Pfizer and St. Jude Medical; grants and personal fees from Lundbeck; and personal fees from AstraZeneca, Canadian Psychiatric Association, Lundbeck Institute, Otsuka, Servier, Canadian Network for Mood and Anxiety Treatments, outside the submitted work. In addition, Dr Lam has a patent Lam Employment Absence and Productivity Scale (LEAPS) issued, a patent Cambridge University Press with royalties paid, a patent Informa Press with royalties paid, and a patent Oxford University Press with royalties paid. Dr Beny Lafer, Dr Hong Qian, Dr Peter H Silverstone, Ms. Nazlin Walji, Dr Angelo da Cunha, Dr Joao Quevedo, Dr Rodrigo Dias, Dr Mauricio Kunz, Dr L Trevor Young and Dr Hubert Wong declare no conflict of interest.
Figures
Comment in
-
Depression following mania.Mol Psychiatry. 2016 Aug;21(8):990. doi: 10.1038/mp.2016.63. Epub 2016 Apr 26. Mol Psychiatry. 2016. PMID: 27113995 No abstract available.
-
Antipsychotic adjunctive therapy to mood stabiliser should be continued for 6 months after remission of a manic episode.Evid Based Ment Health. 2017 Feb;20(1):28. doi: 10.1136/eb-2016-102532. Epub 2016 Dec 30. Evid Based Ment Health. 2017. PMID: 28039171 Free PMC article. No abstract available.
-
Defining the role of SGAs in the long-term treatment of bipolar disorder.Bipolar Disord. 2017 Feb;19(1):65-67. doi: 10.1111/bdi.12472. Bipolar Disord. 2017. PMID: 28346768 No abstract available.
References
-
- Miller DS, Yatham LN, Lam RW. Comparative efficacy of typical and atypical antipsychotics as add-on therapy to mood stabilizers in the treatment of acute mania. J Clin Psychiatry 2001; 62: 975–980. - PubMed
-
- Tohen M, Zarate CA Jr. Antipsychotic agents and bipolar disorder. J Clin Psychiatry 1998; 59: 138–148. - PubMed
-
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15: 1–44. - PubMed
-
- Goodwin GM. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2009; 23: 346–388. - PubMed
-
- Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry 2013; 14: 154–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials