The free heme concentration in healthy human erythrocytes
- PMID: 26460266
- PMCID: PMC4860002
- DOI: 10.1016/j.bcmd.2015.09.003
The free heme concentration in healthy human erythrocytes
Abstract
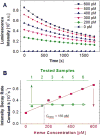
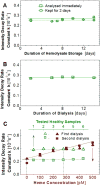
Heme, the prosthetic group of hemoglobin, may be released from its host due to an intrinsic instability of hemoglobin and accumulate in the erythrocytes. Free heme is in the form of hematin (Fe(3+) protoporphyrin IX OH) and follows several pathways of biochemical toxicity to tissues, cells, and organelles since it catalyzes the production of reactive oxygen species. To determine concentration of soluble free heme in human erythrocytes, we develop a new method. We lyse the red blood cells and isolate free heme from hemoglobin by dialysis. We use the heme to reconstitute horseradish peroxidase (HRP) from an excess of the apoenzyme and determine the HRP reaction rate from the evolution of the emitted luminescence. We find that in a population of five healthy adults the average free heme concentration in the erythrocytes is 21±2μM, ca. 100× higher than previously determined. Tests suggest that the lower previous value was due to the use of elevated concentrations of NaCl, which drive hematin precipitation and re-association with apoglobin. We show that the found hematin concentration is significantly higher than estimates based on equilibrium release and the known hematin dimerization. The factors that lead to enhanced heme release remain an open question.
Keywords: Enzymatic analysis; Hematin concentration; Heme release; Hemoglobin instability; Human erythrocytes; Luminescence.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biolumin Chemilumin. 1968;243:465–475. - PubMed
-
- Hargrove MS, Whitaker T, Olson JS, Vali RJ, Mathews AJ. Quaternary structure regulates hemin dissociation from human hemoglobin. J Biol Chem. 1997;272:17385–17389. - PubMed
-
- Light WR, Olson JS. Transmembrane movement of heme. J Biol Chem. 1990;265:15623–15631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

