The mucosal immune system: From dentistry to vaccine development
- PMID: 26460320
- PMCID: PMC4729857
- DOI: 10.2183/pjab.91.423
The mucosal immune system: From dentistry to vaccine development
Abstract
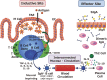
The oral cavity is the beginning of the aero-digestive tract, which is covered by mucosal epithelium continuously under the threat of invasion of pathogens, it is thus protected by the mucosal immune system. In the early phase of our scientific efforts for the demonstration of mucosal immune system, dental science was one of major driving forces due to their foreseeability to use oral immunity for the control of oral diseases. The mucosal immune system is divided functionally into, but interconnected inductive and effector sites. Intestinal Peyer's patches (PPs) are an inductive site containing antigen-sampling M cells and immunocompetent cells required to initiate antigen-specific immune responses. At effector sites, PP-originated antigen-specific IgA B cells become plasma cells to produce polymeric IgA and form secretory IgA by binding to poly-Ig receptor expressed on epithelial cells for protective immunity. The development of new-generation mucosal vaccines, including the rice-based oral vaccine MucoRice, on the basis of the coordinated mucosal immune system is a promising strategy for the control of mucosal infectious diseases.
Figures



References
-
- Takahashi I., Fujihashi K., Kiyono H. (2010) Mucosal regulatory cells in the gastrointestinal tract and periodontium. Periodontol. 2000 54, 247–256. - PubMed
-
- Kiyono, H., Kunisawa, J., McGhee, J.R. and Mestecky, J. (2008) The mucosal immune system. In Fundamental Immunology (ed. Paul, W.E.). Lippincott Williams & Wilkins, Philadelphia, pp. 983–1030.
-
- Kato, T. and Owen, R.L. (2005) Structure and functional intestinal mucosal epithelium. In Mucosal immunology (eds. Mestecky, J., Strober, W., Russell, M.W., Cheroutre, H., Bambrecht, B.N. and Kelsall, B.L.). Elsevier Academic Press, Amsterdam, pp. 131–153.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

