Oseltamivir Population Pharmacokinetics in the Ferret: Model Application for Pharmacokinetic/Pharmacodynamic Study Design
- PMID: 26460484
- PMCID: PMC4603953
- DOI: 10.1371/journal.pone.0138069
Oseltamivir Population Pharmacokinetics in the Ferret: Model Application for Pharmacokinetic/Pharmacodynamic Study Design
Abstract
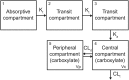
The ferret is a suitable small animal model for preclinical evaluation of efficacy of antiviral drugs against various influenza strains, including highly pathogenic H5N1 viruses. Rigorous pharmacokinetics/pharmacodynamics (PK/PD) assessment of ferret data has not been conducted, perhaps due to insufficient information on oseltamivir PK. Here, based on PK data from several studies on both uninfected and influenza-infected groups (i.e., with influenza A viruses of H5N1 and H3N2 subtypes and an influenza B virus) and several types of anesthesia we developed a population PK model for the active compound oseltamivir carboxylate (OC) in the ferret. The ferret OC population PK model incorporated delayed first-order input, two-compartment distribution, and first-order elimination to successfully describe OC PK. Influenza infection did not affect model parameters, but anesthesia did. The conclusion that OC PK was not influenced by influenza infection must be viewed with caution because the influenza infections in the studies included here resulted in mild clinical symptoms in terms of temperature, body weight, and activity scores. Monte Carlo simulations were used to determine that administration of a 5.08 mg/kg dose of oseltamivir phosphate to ferret every 12 h for 5 days results in the same median OC area under the plasma concentration-time curve 0-12 h (i.e., 3220 mg h/mL) as that observed in humans during steady state at the approved dose of 75 mg twice daily for 5 days. Modeling indicated that PK variability for OC in the ferret model is high, and can be affected by anesthesia. Therefore, for proper interpretation of PK/PD data, sparse PK sampling to allow the OC PK determination in individual animals is important. Another consideration in appropriate design of PK/PD studies is achieving an influenza infection with pronounced clinical symptoms and efficient virus replication, which will allow adequate evaluation of drug effects.
Conflict of interest statement
Figures





References
-
- Funk DJ, Siddiqui F, Wiebe K, Miller RR 3rd, Bautista E, Jimenez E, et al. Practical lessons from the first outbreaks: clinical presentation, obstacles, and management strategies for severe pandemic (pH1N1) 2009 influenza pneumonitis. Crit Care Med. 2010;38: E30–E37. 10.1097/CCM.0b013e3181d10522 - DOI - PubMed
-
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;17: 1872–1879. - PubMed
-
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353: 1363–1373. - PubMed
-
- Smith JR, Ariano RE, Toovey S. The use of antiviral agents for the management of severe influenza. Crit Care Med. 2010;38(Suppl 4): E43–E51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

