A meta-analysis of threats to valid clinical inference in preclinical research of sunitinib
- PMID: 26460544
- PMCID: PMC4600817
- DOI: 10.7554/eLife.08351
A meta-analysis of threats to valid clinical inference in preclinical research of sunitinib
Abstract
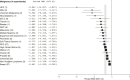
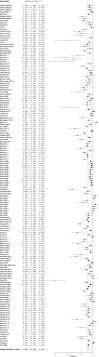
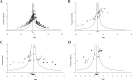
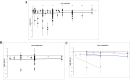
Poor study methodology leads to biased measurement of treatment effects in preclinical research. We used available sunitinib preclinical studies to evaluate relationships between study design and experimental tumor volume effect sizes. We identified published animal efficacy experiments where sunitinib monotherapy was tested for effects on tumor volume. Effect sizes were extracted alongside experimental design elements addressing threats to valid clinical inference. Reported use of practices to address internal validity threats was limited, with no experiments using blinded outcome assessment. Most malignancies were tested in one model only, raising concerns about external validity. We calculate a 45% overestimate of effect size across all malignancies due to potential publication bias. Pooled effect sizes for specific malignancies did not show apparent relationships with effect sizes in clinical trials, and we were unable to detect dose-response relationships. Design and reporting standards represent an opportunity for improving clinical inference.
Keywords: cancer; epidemiology; global health; human biology; medicine; meta-analysis; mouse; preclinical; rat; systematic review.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Anonymous Budgets up at NIH, NCI, and FDA. Cancer Discovery. 2014;4:263. doi: 10.1158/2159-8290.CD-NB2014-016. - DOI - PubMed
-
- Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, Cherrington JM, Pryer NK. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with ‘standard of care’ therapeutic agents for the treatment of breast cancer. Molecular Cancer Therapeutics. 2003;2:1011–1021. - PubMed
-
- Amarasingh S, Macleod MR, Whittle IR. What is the translational efficacy of chemotherapeutic drug research in neuro-oncology? A systematic review and meta-analysis of the efficacy of BCNU and CCNU in animal models of glioma. Journal of Neuro-Oncology. 2009;91:117–125. doi: 10.1007/s11060-008-9697-z. - DOI - PubMed
-
- Amino N, Ideyama Y, Yamano M, Kuromitsu S, Tajinda K, Samizu K, Hisamichi H, Matsuhisa A, Shirasuna K, Kudoh M, Shibasaki M. YM-359445, an orally bioavailable vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor, has highly potent antitumor activity against established tumors. Clinical Cancer Research. 2006;12:1630–1638. doi: 10.1158/1078-0432.CCR-05-2028. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

