Risk of Bias in Reports of In Vivo Research: A Focus for Improvement
- PMID: 26460723
- PMCID: PMC4603955
- DOI: 10.1371/journal.pbio.1002273
Risk of Bias in Reports of In Vivo Research: A Focus for Improvement
Erratum in
-
Correction: Risk of Bias in Reports of In Vivo Research: A Focus for Improvement.PLoS Biol. 2015 Nov 10;13(11):e1002301. doi: 10.1371/journal.pbio.1002301. eCollection 2015 Nov. PLoS Biol. 2015. PMID: 26556632 Free PMC article.
Abstract
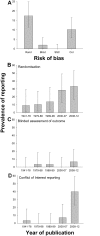
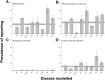
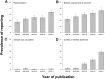
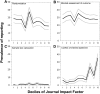
The reliability of experimental findings depends on the rigour of experimental design. Here we show limited reporting of measures to reduce the risk of bias in a random sample of life sciences publications, significantly lower reporting of randomisation in work published in journals of high impact, and very limited reporting of measures to reduce the risk of bias in publications from leading United Kingdom institutions. Ascertainment of differences between institutions might serve both as a measure of research quality and as a tool for institutional efforts to improve research quality.
Conflict of interest statement
MRM is a member of the
Figures
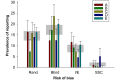
Comment in
-
Poor quality animal studies cause clinical trials to follow false leads.BMJ. 2015 Oct 13;351:h5453. doi: 10.1136/bmj.h5453. BMJ. 2015. PMID: 26468237 No abstract available.
References
-
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 2005. June;25(6):713–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

