The Urine Proteome Profile Is Different in Neuromyelitis Optica Compared to Multiple Sclerosis: A Clinical Proteome Study
- PMID: 26460890
- PMCID: PMC4604198
- DOI: 10.1371/journal.pone.0139659
The Urine Proteome Profile Is Different in Neuromyelitis Optica Compared to Multiple Sclerosis: A Clinical Proteome Study
Abstract
Objectives: Inflammatory demyelinating diseases of the CNS comprise a broad spectrum of diseases like neuromyelitis optica (NMO), NMO spectrum disorders (NMO-SD) and multiple sclerosis (MS). Despite clear classification criteria, differentiation can be difficult. We hypothesized that the urine proteome may differentiate NMO from MS.
Methods: The proteins in urine samples from anti-aquaporin 4 (AQP4) seropositive NMO/NMO-SD patients (n = 32), patients with MS (n = 46) and healthy subjects (HS, n = 31) were examined by quantitative liquid chromatography-tandem mass spectrometry (LC-MS/MS) after trypsin digestion and iTRAQ labelling. Immunoglobulins (Ig) in the urine were validated by nephelometry in an independent cohort (n = 9-10 pr. groups).
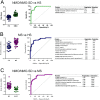
Results: The analysis identified a total of 1112 different proteins of which 333 were shared by all 109 subjects. Cluster analysis revealed differences in the urine proteome of NMO/NMO-SD compared to HS and MS. Principal component analysis also suggested that the NMO/NMO-SD proteome profile was useful for classification. Multivariate regression analysis revealed a 3-protein profile for the NMO/NMO-SD versus HS discrimination, a 6-protein profile for NMO/NMO-SD versus MS discrimination and an 11-protein profile for MS versus HS discrimination. All protein panels yielded highly significant ROC curves (AUC in all cases >0.85, p≤0.0002). Nephelometry confirmed the presence of increased Ig-light chains in the urine of patients with NMO/NMO-SD.
Conclusion: The urine proteome profile of patients with NMO/NMO-SD is different from MS and HS. This may reflect differences in the pathogenesis of NMO/NMO-SD versus MS and suggests that urine may be a potential source of biomarkers differentiating NMO/NMO-SD from MS.
Conflict of interest statement
Figures




References
-
- Illes Z. Pathogenesis, diagnosis and treatment of neuromyelitis optica: Changing concept of an old disease. Clinical and Experimental Neuroimmunology. 2010;1(3):103–23. 10.1111/j.1759-1961.2010.00011.x - DOI
-
- Sato DK, Callegaro D, Lana-Peixoto MA, Nakashima I, Fujihara K. Seronegative Neuromyelitis Optica Spectrum–-the challenges on disease definition and pathogenesis. Arquivos de neuro-psiquiatria. 2014;72(6):445–50. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

