Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin
- PMID: 26461048
- PMCID: PMC4633820
- DOI: 10.1038/ncomms9545
Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin
Abstract
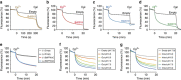
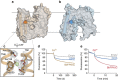
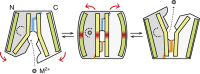
In vertebrates, the iron exporter ferroportin releases Fe(2+) from cells into plasma, thereby maintaining iron homeostasis. The transport activity of ferroportin is suppressed by the peptide hormone hepcidin, which exhibits upregulated expression in chronic inflammation, causing iron-restrictive anaemia. However, due to the lack of structural information about ferroportin, the mechanisms of its iron transport and hepcidin-mediated regulation remain largely elusive. Here we report the crystal structures of a putative bacterial homologue of ferroportin, BbFPN, in both the outward- and inward-facing states. Despite undetectable sequence similarity, BbFPN adopts the major facilitator superfamily fold. A comparison of the two structures reveals that BbFPN undergoes an intra-domain conformational rearrangement during the transport cycle. We identify a substrate metal-binding site, based on structural and mutational analyses. Furthermore, the BbFPN structures suggest that a predicted hepcidin-binding site of ferroportin is located within its central cavity. Thus, BbFPN may be a valuable structural model for iron homeostasis regulation by ferroportin.
Figures




References
-
- McKie A. T. et al.. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 (2000). - PubMed
-
- Donovan A. et al.. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 (2000). - PubMed
-
- Abboud S. & Haile D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 (2000). - PubMed
-
- Yang F. et al.. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J. Biol. Chem. 277, 39786–39791 (2002). - PubMed
-
- Hentze M. W., Muckenthaler M. U., Galy B. & Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

