Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments
- PMID: 26461445
- PMCID: PMC4654682
- DOI: 10.1038/nmat4444
Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments
Abstract
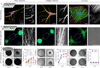
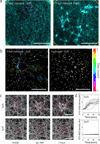
To investigate how cells sense stiffness in settings structurally similar to native extracellular matrices, we designed a synthetic fibrous material with tunable mechanics and user-defined architecture. In contrast to flat hydrogel surfaces, these fibrous materials recapitulated cell-matrix interactions observed with collagen matrices including stellate cell morphologies, cell-mediated realignment of fibres, and bulk contraction of the material. Increasing the stiffness of flat hydrogel surfaces induced mesenchymal stem cell spreading and proliferation; however, increasing fibre stiffness instead suppressed spreading and proliferation for certain network architectures. Lower fibre stiffness permitted active cellular forces to recruit nearby fibres, dynamically increasing ligand density at the cell surface and promoting the formation of focal adhesions and related signalling. These studies demonstrate a departure from the well-described relationship between material stiffness and spreading established with hydrogel surfaces, and introduce fibre recruitment as a previously undescribed mechanism by which cells probe and respond to mechanics in fibrillar matrices.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- HL124322/HL/NHLBI NIH HHS/United States
- EB000262/EB/NIBIB NIH HHS/United States
- EB014691/EB/NIBIB NIH HHS/United States
- HL115553/HL/NHLBI NIH HHS/United States
- P41 EB001046/EB/NIBIB NIH HHS/United States
- R01 AR056624/AR/NIAMS NIH HHS/United States
- R01 HL115553/HL/NHLBI NIH HHS/United States
- R00 HL124322/HL/NHLBI NIH HHS/United States
- AR056624/AR/NIAMS NIH HHS/United States
- U54 CA193417/CA/NCI NIH HHS/United States
- R01 EB000262/EB/NIBIB NIH HHS/United States
- F32 EB014691/EB/NIBIB NIH HHS/United States
- GM74048/GM/NIGMS NIH HHS/United States
- K99 HL124322/HL/NHLBI NIH HHS/United States
- EB001046/EB/NIBIB NIH HHS/United States
- R01 EB017753/EB/NIBIB NIH HHS/United States
- R01 GM074048/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

