Standardised Resting Time Prior to Blood Sampling and Diurnal Variation Associated with Risk of Patient Misclassification: Results from Selected Biochemical Components
- PMID: 26461522
- PMCID: PMC4604193
- DOI: 10.1371/journal.pone.0140475
Standardised Resting Time Prior to Blood Sampling and Diurnal Variation Associated with Risk of Patient Misclassification: Results from Selected Biochemical Components
Abstract
Background: According to current recommendations, blood samples should be taken in the morning after 15 minutes' resting time. Some components exhibit diurnal variation and in response to pressures to expand opening hours and reduce waiting time, the aims of this study were to investigate the impact of resting time prior to blood sampling and diurnal variation on biochemical components, including albumin, thyrotropin (TSH), total calcium and sodium in plasma.
Methods: All patients referred to an outpatient clinic for blood sampling were included in the period Nov 2011 until June 2014 (opening hours: 7am-3pm). Each patient's arrival time and time of blood sampling were registered. The impact of resting time and the time of day for all components was analysed using simple linear regression. The "maximum allowable bias" was used as quality indicator for the change in reference interval.
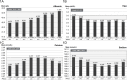
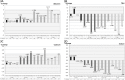
Results: Significant diurnal variation was found for albumin (n = 15,544; p<2×10-16), TSH (n = 20,019; p<2×10-16), calcium (n = 13,588; p = 2.8×10-12) and sodium (n = 51,917; p<2×10-16). Further significant influence for resting time was found for albumin (p = 2.6×10-4), TSH (p = 0.004), calcium (p = 8.9×10-7) and sodium (p = 8.7×10-16). Only TSH and albumin were clinically significantly influenced by diurnal variation. Resting time had no clinically significant effect.
Conclusions: We found no need for resting 15 minutes prior to blood sampling. However, diurnal variation was found to have a significant and considerable impact on TSH and, to a minor degree, albumin. This has to be taken into account to ensure that reference intervals provided by the laboratory are valid on a 24-hour basis.
Conflict of interest statement
Figures

References
-
- Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin Chim Acta 2014;432:33–37. 10.1016/j.cca.2013.11.008 - DOI - PubMed
-
- Weeke J, Gundersen HJG. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol 1978;89:659–672. - PubMed
-
- Jensen E, Blaabjerg O, Petersen PH, Hegedüs L. Sampling time is important but may be overlooked in establishment and use of thyroid-stimulating hormone reference intervals [Letter]. Clin Chem 2007;53:355–356. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

