Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells
- PMID: 26461813
- PMCID: PMC4602195
- DOI: 10.1038/srep15042
Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells
Abstract
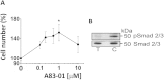
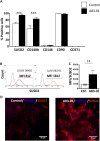
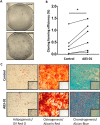
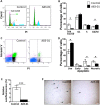
Human endometrial MSC (eMSC) are a novel source of MSC easily harvested from the highly regenerative uterine lining. We have developed protocols for eMSC isolation from single cell suspensions using magnetic bead-sorting using a perivascular marker antibody to SUSD2 and culture expansion in serum free medium (SFM). Similar to other MSC, eMSC spontaneously differentiate into fibroblasts during culture expansion decreasing their purity and efficacy. The aim of this study was to determine if A83-01, a TGF-β receptor inhibitor prevents eMSC differentiation in culture. SUSD2(+) eMSC were cultured in SFM with bFGF/EGF in 5% O2/5% CO2. At passage 6, eMSC were incubated with or without A83-01 for 7 days, then analysed for MSC properties. A83-01 dose dependently promoted SUSD2(+) eMSC proliferation and blocked apoptosis via the SMAD 2/3 pathway. Fewer A83-01 treated cells were autofluorescent or stained with β-galactosidase, indicating reduced senescence. A83-01-treated cells had higher cloning efficiency, differentiated into mesodermal lineages and expressed MSC phenotypic markers. These data suggest that A83-01 maintains SUSD2(+) eMSC stemness, promoting proliferation by blocking senescence and apoptosis in late passage cultures through binding to TGF-β receptors. Small molecules such as A83-01 may enable the expansion of undifferentiated MSC for use in tissue engineering and cell-based therapies.
Figures

References
-
- Friedenstein A. J. et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 2, 83–92 (1974). - PubMed
-
- Pittenger M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999). - PubMed
-
- Caplan A. I. & Bruder S. P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends in molecular medicine 7, 259–264 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

