NET amyloidogenic backbone in human activated neutrophils
- PMID: 26462606
- PMCID: PMC4750596
- DOI: 10.1111/cei.12730
NET amyloidogenic backbone in human activated neutrophils
Abstract
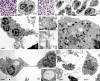
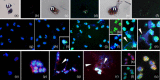
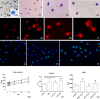
Activated human neutrophils produce a fibrillar DNA network [neutrophil extracellular traps (NETs)] for entrapping and killing bacteria, fungi, protozoa and viruses. Our results suggest that the neutrophil extracellular traps show a resistant amyloidogenic backbone utilized for addressing reputed proteins and DNA against the non-self. The formation of amyloid fibrils in neutrophils is regulated by the imbalance of reactive oxygen species (ROS) in the cytoplasm. The intensity and source of the ROS signal is determinant for promoting stress-associated responses such as amyloidogenesis and closely related events: autophagy, exosome release, activation of the adrenocorticotrophin hormone/α-melanocyte-stimulating hormone (ACTH/α-MSH) loop and synthesis of specific cytokines. These interconnected responses in human activated neutrophils, that have been evaluated from a morphofunctional and quantitative viewpoint, represent primitive, but potent, innate defence mechanisms. In invertebrates, circulating phagocytic immune cells, when activated, show responses similar to those described previously for activated human neutrophils. Invertebrate cells within endoplasmic reticulum cisternae produce a fibrillar material which is then assembled into an amyloidogenic scaffold utilized to convey melanin close to the invader. These findings, in consideration to the critical role played by NET in the development of several pathologies, could explain the structural resistance of these scaffolds and could provide the basis for developing new diagnostic and therapeutic approaches in immunomediated diseases in which the innate branch of the immune system has a pivotal role.
Keywords: ACTH axis; ROS evaluation; amyloidogenesis; exosomes; neutrophil extracellular trap.
© 2015 British Society for Immunology.
Figures


References
-
- Borregaard N, Cowland J. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997; 89:3503–21. - PubMed
-
- Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 2007; 5:577–82. - PubMed
-
- Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009; 30:513–21. - PubMed
-
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptative immunity. Nat Rev Immunol 2011; 11:519–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

