How astrocyte networks may contribute to cerebral metabolite clearance
- PMID: 26463008
- PMCID: PMC4604494
- DOI: 10.1038/srep15024
How astrocyte networks may contribute to cerebral metabolite clearance
Abstract
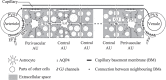
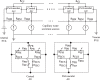
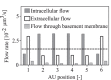
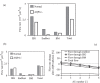
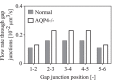
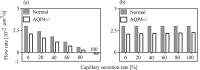
The brain possesses an intricate network of interconnected fluid pathways that are vital to the maintenance of its homeostasis. With diffusion being the main mode of solute transport in cerebral tissue, it is not clear how bulk flow through these pathways is involved in the removal of metabolites. In this computational study, we show that networks of astrocytes may contribute to the passage of solutes between tissue and paravascular spaces (PVS) by serving as low resistance pathways to bulk water flow. The astrocyte networks are connected through aquaporin-4 (AQP4) water channels with a parallel, extracellular route carrying metabolites. Inhibition of the intracellular route by deletion of AQP4 causes a reduction of bulk flow between tissue and PVS, leading to reduced metabolite clearance into the venous PVS or, as observed in animal studies, a reduction of tracer influx from arterial PVS into the brain tissue.
Figures


References
-
- Kurtcuoglu V. et al. Computational investigation of subject-specific cerebrospinal fluid flow in the third ventricle and aqueduct of Sylvius. J Biomech 40, 1235–1245 (2007). - PubMed
-
- Carare R. O. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34, 131–144 (2008). - PubMed
-
- Cserr H. & Patlak C. Secretion and bulk flow of interstitial fluid. In: Physiology and pharmacology of the blood-brain barrier (ed^(eds). Springer (1992).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

