Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system
- PMID: 26463117
- PMCID: PMC4717280
- DOI: 10.1111/acel.12405
Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system
Abstract
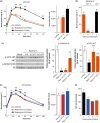
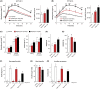
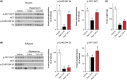
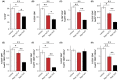
Inhibition of the mechanistic target of rapamycin (mTOR) signaling pathway by the FDA-approved drug rapamycin has been shown to promote lifespan and delay age-related diseases in model organisms including mice. Unfortunately, rapamycin has potentially serious side effects in humans, including glucose intolerance and immunosuppression, which may preclude the long-term prophylactic use of rapamycin as a therapy for age-related diseases. While the beneficial effects of rapamycin are largely mediated by the inhibition of mTOR complex 1 (mTORC1), which is acutely sensitive to rapamycin, many of the negative side effects are mediated by the inhibition of a second mTOR-containing complex, mTORC2, which is much less sensitive to rapamycin. We hypothesized that different rapamycin dosing schedules or the use of FDA-approved rapamycin analogs with different pharmacokinetics might expand the therapeutic window of rapamycin by more specifically targeting mTORC1. Here, we identified an intermittent rapamycin dosing schedule with minimal effects on glucose tolerance, and we find that this schedule has a reduced impact on pyruvate tolerance, fasting glucose and insulin levels, beta cell function, and the immune system compared to daily rapamycin treatment. Further, we find that the FDA-approved rapamycin analogs everolimus and temsirolimus efficiently inhibit mTORC1 while having a reduced impact on glucose and pyruvate tolerance. Our results suggest that many of the negative side effects of rapamycin treatment can be mitigated through intermittent dosing or the use of rapamycin analogs.
Keywords: aging; mechanistic target of rapamycin; mice; rapamycin.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

References
-
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV (2011) Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

