Dysregulation of Plasmalogen Homeostasis Impairs Cholesterol Biosynthesis
- PMID: 26463208
- PMCID: PMC4661398
- DOI: 10.1074/jbc.M115.656983
Dysregulation of Plasmalogen Homeostasis Impairs Cholesterol Biosynthesis
Abstract
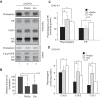
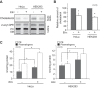
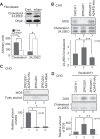
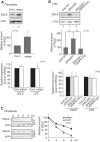
Plasmalogen biosynthesis is regulated by modulating fatty acyl-CoA reductase 1 stability in a manner dependent on cellular plasmalogen level. However, physiological significance of the regulation of plasmalogen biosynthesis remains unknown. Here we show that elevation of the cellular plasmalogen level reduces cholesterol biosynthesis without affecting the isoprenylation of proteins such as Rab and Pex19p. Analysis of intermediate metabolites in cholesterol biosynthesis suggests that the first oxidative step in cholesterol biosynthesis catalyzed by squalene monooxygenase (SQLE), an important regulator downstream HMG-CoA reductase in cholesterol synthesis, is reduced by degradation of SQLE upon elevation of cellular plasmalogen level. By contrast, the defect of plasmalogen synthesis causes elevation of SQLE expression, resulting in the reduction of 2,3-epoxysqualene required for cholesterol synthesis, hence implying a novel physiological consequence of the regulation of plasmalogen biosynthesis.
Keywords: cell metabolism; cholesterol; endoplasmic reticulum (ER); peroxisome; plasmalogen; squalen; squalene monooxygenase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Steinberg S. J., Dodt G., Raymond G. V., Braverman N. E., Moser A. B., and Moser H. W. (2006) Peroxisome biogenesis disorders. Biochim. Biophys. Acta 1763, 1733–1748 - PubMed
-
- Braverman N. E., and Moser A. B. (2012) Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 1822, 1442–1452 - PubMed
-
- Munn N. J., Arnio E., Liu D., Zoeller R. A., and Liscum L. (2003) Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J. Lipid Res. 44, 182–192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

