Do Multidisciplinary Team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience
- PMID: 26463599
- PMCID: PMC4604766
- DOI: 10.1186/s12885-015-1683-1
Do Multidisciplinary Team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience
Abstract
Background: MDT (multidisciplinary team) meetings are considered an essential component of care for patients with cancer. However there is remarkably little direct evidence that such meetings improve outcomes. We assessed whether or not MDT (multidisciplinary team) processes influenced survival in a cohort of patients with colorectal cancer.
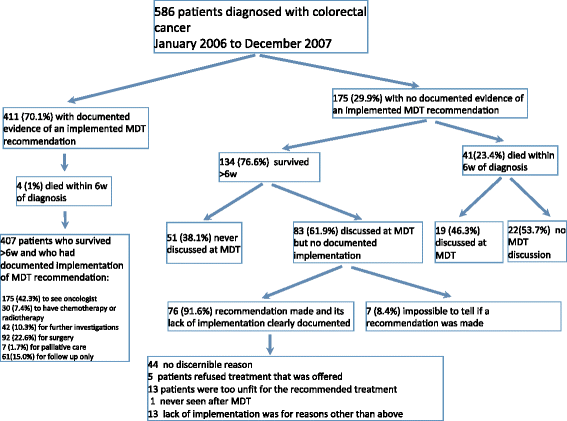
Methods: Observational study of a population-based cohort of 586 consecutive patients with colorectal cancer diagnosed in Tayside (Scotland) during 2006 and 2007.
Results: Recommendations from MDT meetings were implemented in 411/586 (70.1 %) of patients, the MDT+ group. The remaining175/586 (29.9 %) were either never discussed at an MDT, or recommendations were not implemented, MDT- group. The 5-year cause-specific survival (CSS) rates were 63.1 % (MDT+) and 48.2 % (MDT-), p < 0.0001. In analysis confined to patients who survived >6 weeks after diagnosis, the rates were 63.2 % (MDT+) and 57.7 % (MDT-), p = 0.064. The adjusted hazard rate (HR) for death from colorectal cancer was 0.73 (0.53 to 1.00, p = 0.047) in the MDT+ group compared to the MDT- group, in patients surviving >6 weeks the adjusted HR was 1.00 (0.70 to 1.42, p = 0.987). Any benefit from the MDT process was largely confined to patients with advanced disease: adjusted HR (early) 1.32 (0.69 to 2.49, p = 0.401); adjusted HR(advanced) 0.65 (0.45 to 0.96, p = 0.031).
Conclusions: Adequate MDT processes are associated with improved survival for patients with colorectal cancer. However, some of this effect may be more apparent than real - simply reflecting selection bias. The MDT process predominantly benefits the 40 % of patients who present with advanced disease and conveys little demonstrable advantage to patients with early tumours. These results call into question the current belief that all new patients with colorectal cancer should be discussed at an MDT meeting.
Figures
References
-
- Calman K, Hine D. A policy framework for commissioning cancer services: a report by the expert advisory group on cancer to the chief medical officers of England and wales. 1995.
-
- Department of Health . The NHS cancer plan, A plan for investment, A plan for reform. London: Department of Health; 2000.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous