Polymeric Nanostructures for Imaging and Therapy
- PMID: 26463640
- PMCID: PMC4610256
- DOI: 10.1021/acs.chemrev.5b00135
Polymeric Nanostructures for Imaging and Therapy
Figures













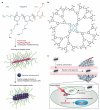
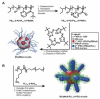

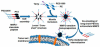








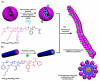
References
-
- Toumey C. Plenty of room, plenty of history. Nat. Nanotechnol. 2009;4:783–784. - PubMed
-
- Taniguchi N. On the basic concept of nano-technology; Proc. Intl. Conf. Prod. Eng. Tokyo, Part II, Japan Society of Precision Engineering; 1974.
-
- Devadasu VR, Bhardwaj V, Kumar MN. Can controversial nanotechnology promise drug delivery? Chem. Rev. 2013;113:1686–1735. - PubMed
-
- Brambilla D, Luciani P, Leroux JC. Breakthrough discoveries in drug delivery technologies: the next 30 years. J. Control. Release. 2014;190:9–14. - PubMed
-
- Pahlm O, Wagner G. Multimodal Cardiovascular Imaging: Principles and Clinical Applications. McGraw-Hill; New York: 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

