The monoamine stabilizer (-)-OSU6162 counteracts downregulated dopamine output in the nucleus accumbens of long-term drinking Wistar rats
- PMID: 26464265
- PMCID: PMC5057338
- DOI: 10.1111/adb.12304
The monoamine stabilizer (-)-OSU6162 counteracts downregulated dopamine output in the nucleus accumbens of long-term drinking Wistar rats
Abstract
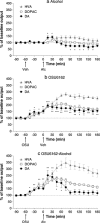
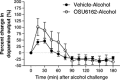
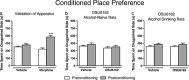
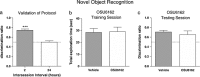
We recently established that the monoamine stabilizer (-)-OSU6162 (OSU6162) decreased voluntary alcohol-mediated behaviors, including alcohol intake and cue/priming-induced reinstatement, in long-term drinking rats, while blunting alcohol-induced dopamine output in the nucleus accumbens (NAc) of alcohol-naïve rats. Therefore, we hypothesized that OSU6162 attenuates alcohol-mediated behaviors by blunting alcohol's rewarding effects. Here, we evaluated the effects of long-term drinking and OSU6162 treatment (30 mg/kg, sc) on basal and alcohol-induced (2.5 g/kg, ip) NAc dopamine outputs in Wistar rats after 10 months of intermittent access to 20% alcohol. The results showed that basal and alcohol-induced NAc dopamine outputs were significantly lower in long-term drinking rats, compared with alcohol-naïve rats. In the long-term drinking rats, OSU6162 slowly increased and maintained the dopamine output significantly elevated compared with baseline for at least 4 hours. Furthermore, OSU6162 pre-treatment did not blunt the alcohol-induced output in the long-term drinking rats, a finding that contrasted with our previous results in alcohol-naïve rats. Finally, OSU6162 did not induce conditioned place preference (CPP) in either long-term drinking or alcohol-naïve rats, indicating that OSU6162 has no reinforcing properties. To verify that the CPP results were not due to memory acquisition impairment, we demonstrated that OSU6162 did not affect novel object recognition. In conclusion, these results indicate that OSU6162 attenuates alcohol-mediated behaviors by counteracting NAc dopamine deficits in long-term drinking rats and that OSU6162 is not rewarding on its own. Together with OSU6162's beneficial side-effect profile, the present study merits evaluation of OSU6162's clinical efficacy to attenuate alcohol use in alcohol-dependent patients.
Keywords: Alcohol dependence; condition place preference; ethanol; medication development; microdialysis.
© 2015 The Authors.Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures

References
-
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. - PubMed
-
- Barak S, Carnicella S, Yowell QV, Ron D (2011) Glial cell line‐derived neurotrophic factor reverses alcohol‐induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 31:9885–9894. - PMC - PubMed
-
- Bass CE, Grinevich VP, Gioia D, Day‐Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA (2013) Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self‐administration. Frontiers in Behavioral Neuroscience 7:173. - PMC - PubMed
-
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

