Comparative morphology of the nervous system in three phylactolaemate bryozoans
- PMID: 26464575
- PMCID: PMC4603689
- DOI: 10.1186/s12983-015-0112-2
Comparative morphology of the nervous system in three phylactolaemate bryozoans
Abstract
Background: Though some elements of the bryozoan nervous system were discovered 180 years ago, few studies of their neuromorphology have been undertaken since that time. As a result the general picture of the bryozoan nervous system structure is incomplete in respect of details and fragmentary in respect of taxonomic coverage.
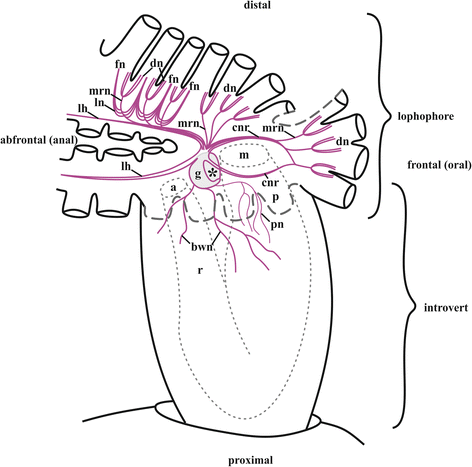
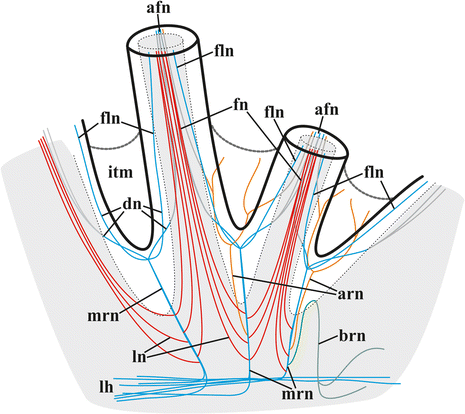
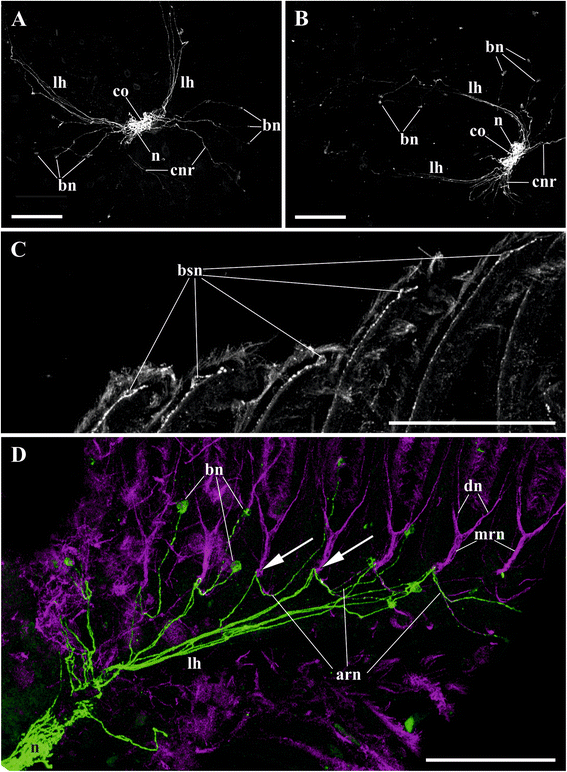
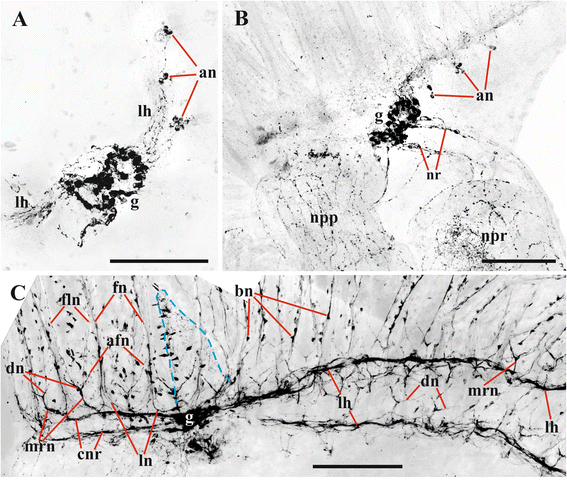
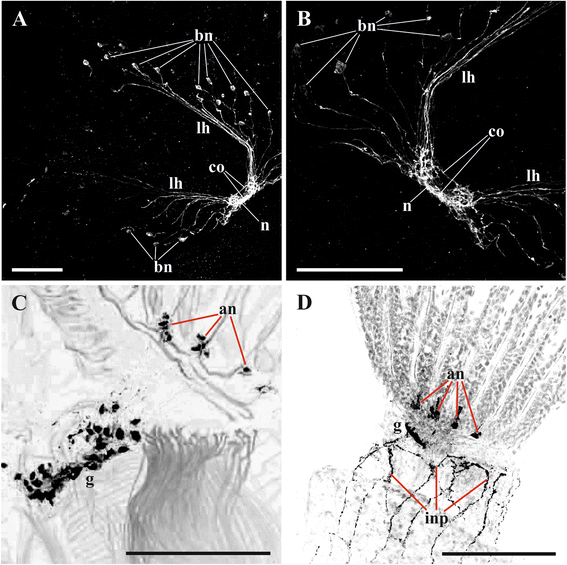
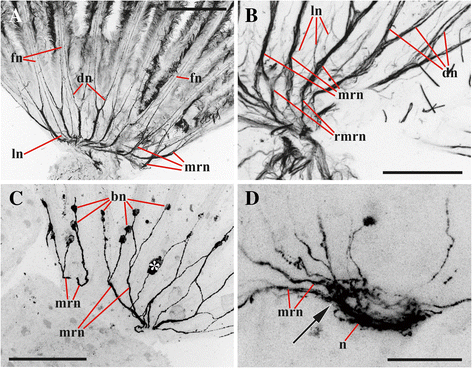
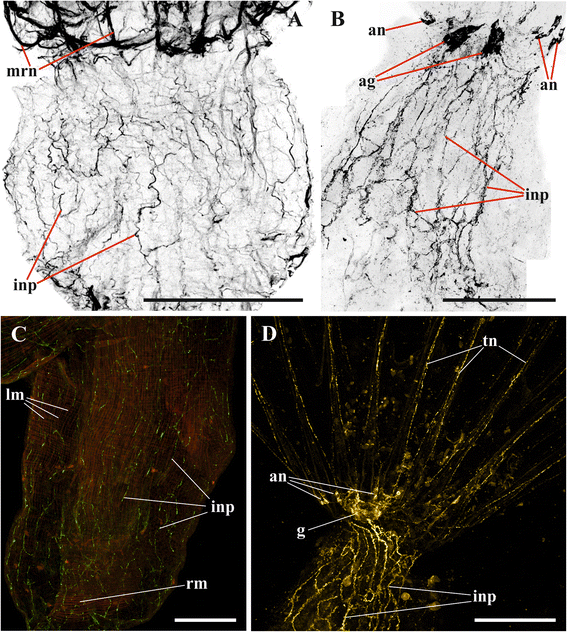
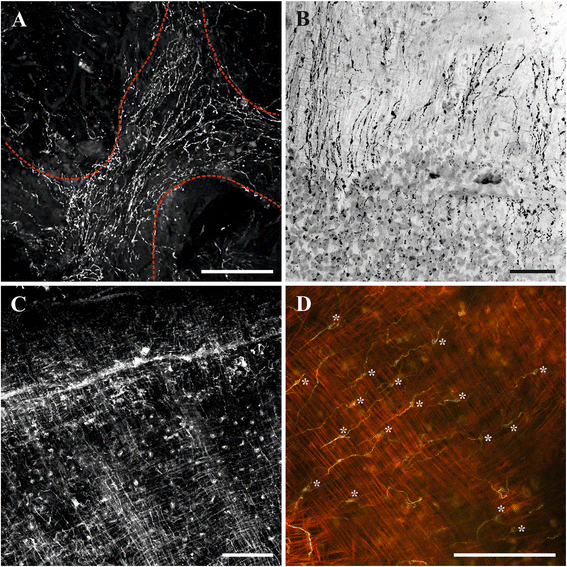
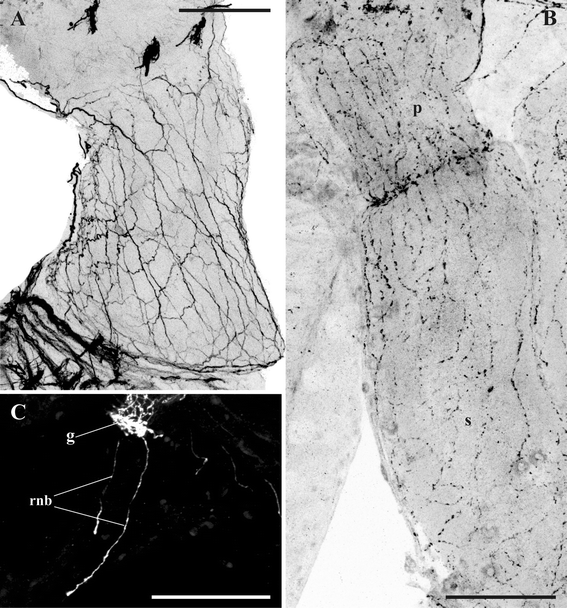
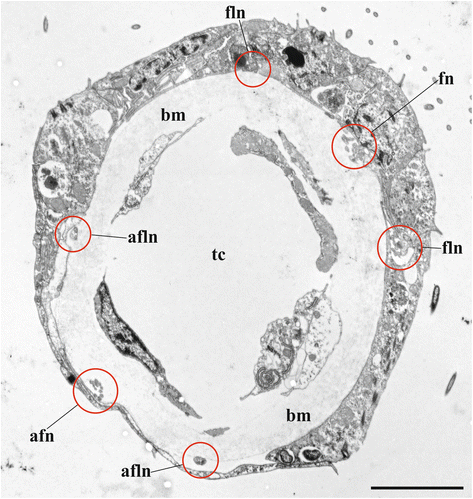
Results: The nervous system of three common European freshwater bryozoans - Cristatella mucedo, Plumatella repens (both with a horseshoe-shaped lophophore) and Fredericella sultana (with a circular lophophore) had numerous differences in the details of the structure but the general neuroarchitecture is similar. The nervous system of the zooid consists of the cerebral ganglion, a circumpharyngeal ring and lophophoral nerve tracts (horns), both sending numerous nerves to the tentacles, and the nerve plexuses of the body wall and of the gut. A number of the important details (distal branching of the additional radial nerve, pattern of distribution of nerve cells and neurites in the ganglion, etc.) were described for the first time. The number and position of the tentacle nerves in Cristatella mucedo was ascertained and suggestions about their function were made. The revealed distribution of various neuromediators in the nervous system allowed us to suggest functional affinities of some major nerves.
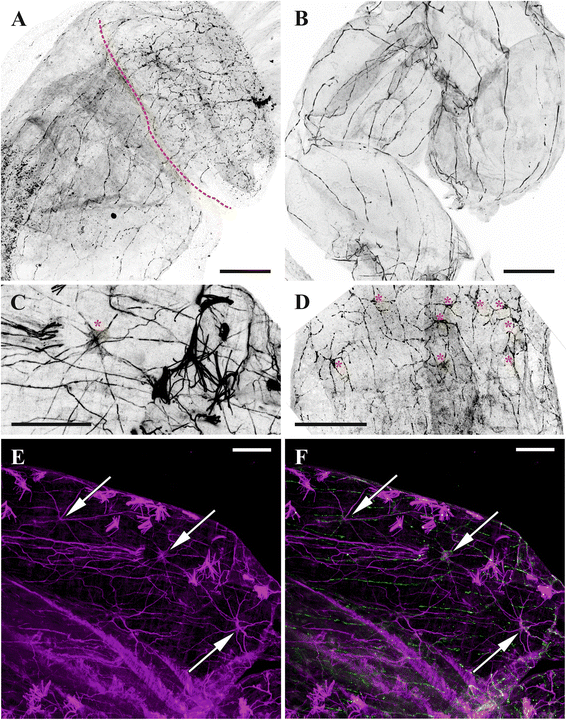
Conclusions: Despite the basic similarity, both the ganglion and the lophophore nervous system in Phylactolaemata have a more complex structure than in marine bryozoans (classes Gymnolaemata and Stenolaemata). First of all, their neuronal network has a denser and more complex branching pattern: most phylactolaemates have two large nerve tracts associated with lophophore arms, they have more nerves in the tentacles, additional and basal branches emitting from the main radial nerves, etc. This, in part, can be explained by the horseshoe shape of the lophophore and a larger size of the polypide in freshwater species. The structure of the nervous system in Fredericella sultana suggests that it underwent a secondary simplification following the reduction of the lophophore arms. Colony locomotion in Cristatella mucedo is based on co-ordinated activity of two perpendicular muscle layers of the sole and the plexus of motor neurons sandwiched between them. The trigger of this activity and the co-ordination mechanism remain enigmatic.
Keywords: Bryozoa; Immunohistochemistry; Nervous system; Neuromediators; Phylactolaemata.
Figures






References
-
- Ryland JS. Bryozoa: an introductory overview. In: Wöss, E, editor. Moostiere (Bryozoa). Linz: Oberösterreichisches Landesmuseum; Denisia; 2005, NS 28, 16:9–20.
-
- Reed CG. Bryozoa. In: Giese AC, Pearse JS, Pearse VB, editors. Reproduction of Marine Invertebrates – Echinoderms and Lophophorates. Pacific Grove: The Boxwood Press; 1991. pp. 85–245.
-
- Mukai H, Terakado K, Reed CG. Bryozoa. In: Harrison FW, editor. Microscopic Anatomy of Invertebrates. New York: Wiley-Liss; 1997. pp. 45–206.
-
- Allman G. On the homology of the organs of the Tunicata and of the Polyzoa. Trans R Irish Acad. 1852;22:275–289.
-
- Hyman LH. The invertebrates: Smaller coelomate groups. New York: McGraw-Hill; 1959.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

